Question
Question: Identify the end-product [D] formed when solution salicylate undergoes the following series of react...
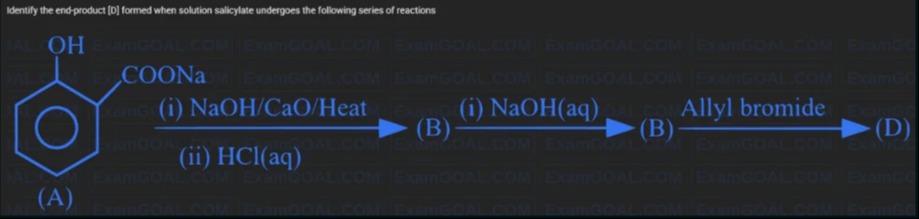
Identify the end-product [D] formed when solution salicylate undergoes the following series of reactions
Answer
Allyl phenyl ether (phenyl allyl ether)
Explanation
Solution
Step 1: Decarboxylation
Under NaOH/CaO and heat, the salicylate ion loses CO₂ to give phenoxide.
\ceC6H4(OH)COO−−>[NaOH/CaO,;Δ]C6H5O−+CO2
Step 2: Acidification
Acid workup (HCl) converts phenoxide to phenol.
\ceC6H5O−+HCl−>C6H5OH+Cl−
Step 3: Williamson Ether Synthesis
Phenol in NaOH(aq) forms phenoxide again, which reacts with allyl bromide via S_N2 to yield allyl phenyl ether.
\ceC6H5O−+CH2=CHCH2Br−>C6H5OCH2CH=CH2+Br−
Key point: Phenol undergoes Williamson ether synthesis with allyl bromide to give the allyl ether.
