Question
Question: Identify the correct statements regarding structure of diborane: (This question has multiple corre...
Identify the correct statements regarding structure of diborane:
(This question has multiple correct options)
A. There are two bridging hydrogen atoms
B. Each boron atom forms four bonds
C. The hydrogen atoms are not in the same plane
D. Each boron atom is in sp3 hybridized state
Solution
Hint: Diborane has bridge bonding and it has 3C−2e− bond. The Boron element has 1s22s22p1 configuration and its valency is 3. Configuration of boron in borane is sp2.
Step by step solution:
Diborane is formed by dimerisation of borane:
BH3DimerisationB2H6
Diborane has non-planar structure.
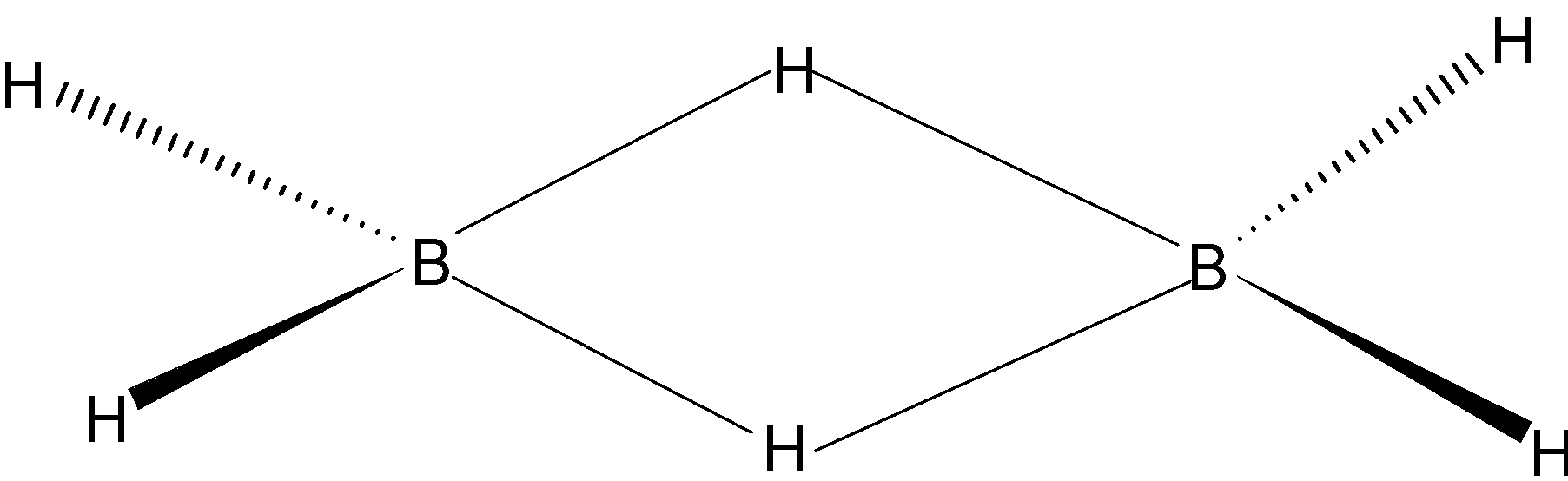
“A” is correct, because in diborane there are 6 hydrogen atoms in which 2 hydrogens are bridged and the other 4 are in the same plane.
“B” is correct, because each boron forms four bonds, two bonds with two bridged hydrogen (3C−2e−) and two bonds (2C−2e−) with the same planar hydrogen.
“C” is correct, because 4 hydrogens are in the same plane and two bridged hydrogens are there to reduce the bond pair – bond pair repulsion.
“D” is correct, because this molecule has a total 12 valance electrons, 3 from boron and 6 from hydrogen. Here, 8 e- are used in normal 2C−2e− bonds and other 4valence electrons are shared by remaining 2 hydrogens and borons for making banana bonds. Each atom contributes one orbital to form 3C−2e− bond. Boron uses its vacant p orbital, so its configuration is sp3.
Note: 3C−2e− bond is also known as banana bond/p-seudo bond/tau bond. It is a non-planar structure, which we can understand from the configuration of boron (sp3). Banana bonds are covalent in nature, so there are 2 electrons which are shared by 3 centres.
