Question
Question: Identify the correct order of rate of reaction for the compounds given below:...
Identify the correct order of rate of reaction for the compounds given below:
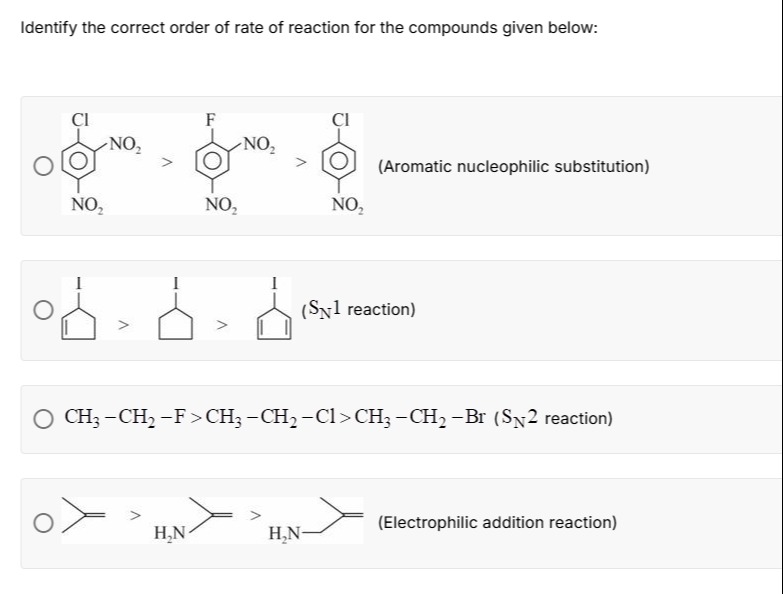
CH3-CH2-F>CH3-CH2-Cl>CH3-CH2-Br (SN2 reaction)
All the given orders are incorrect based on standard organic chemistry principles. The question likely contains an error or requires specific context not provided.
Solution
Let's analyze each reaction type:
-
Aromatic Nucleophilic Substitution (SNAr):
The rate-determining step involves the formation of a Meisenheimer complex. Generally, the leaving group ability order is F > Cl > Br > I due to fluorine's high electronegativity stabilizing the transition state. Therefore, the given order is incorrect. -
SN1 Reaction:
SN1 reactions proceed via carbocation intermediates. Alkyl carbocations are more stable than vinyl carbocations. Therefore, the given order is incorrect. -
SN2 Reaction:
SN2 reaction rates depend on leaving group ability, which follows the order I- > Br- > Cl- > F-. Therefore, the given order is incorrect. -
Electrophilic Addition Reaction:
The reaction rate depends on carbocation stability. Vinylamine (CH₂=CH-NH₂) forms a highly resonance-stabilized carbocation. Isobutylene ((CH₃)₂C=CH₂) forms a tertiary carbocation. Allylamine (CH₂=CH-CH₂-NH₂) forms a secondary allylic carbocation. The stability order is typically Vinylamine > Isobutylene > Allylamine. However, under acidic conditions, vinylamine can be protonated, deactivating it. Even with this consideration, the given order is not generally correct without specific contextual assumptions.
Conclusion: All provided orders are incorrect based on general organic chemistry principles. The question might contain an error or rely on specific, unstated conditions.
