Question
Question: Identify the correct option According to \(CIP\) sequence rule, the correct arrangement in order o...
Identify the correct option
According to CIP sequence rule, the correct arrangement in order of decreasing priority is:
A. −OH>CH2OH>−CHO>−COOH
B. −OH>COOH>−CHO>−CH2OH
C. −COOH>OH>−COH>−CH2OH
D. COOH>−CHO>−CH2OH>−OH
Solution
The higher the atomic number of the immediate substituent atom, the higher the priority.
For example:
H−<C−<N−<O−<Cl−
Complete step by step answer:
-The CIP rule is a standard process used in organic chemistry to completely and unequivocally name a stereoisomer of a molecule.
-Priority is numerically assigned as 1,2,3 and 4 where smaller the number higher will be the priority. So, 1 has the highest priority and 4 has the lowest priority.
Rules for giving the priority:
Rule1: Atoms having greater atomic number is given more priority
−OH⇒First
Atomic number of 0 is 8.
Rule 2: If 1st atom is the same then, see 2nd atom and apply Rule 1.
Rule 3: For multiple bond
I
II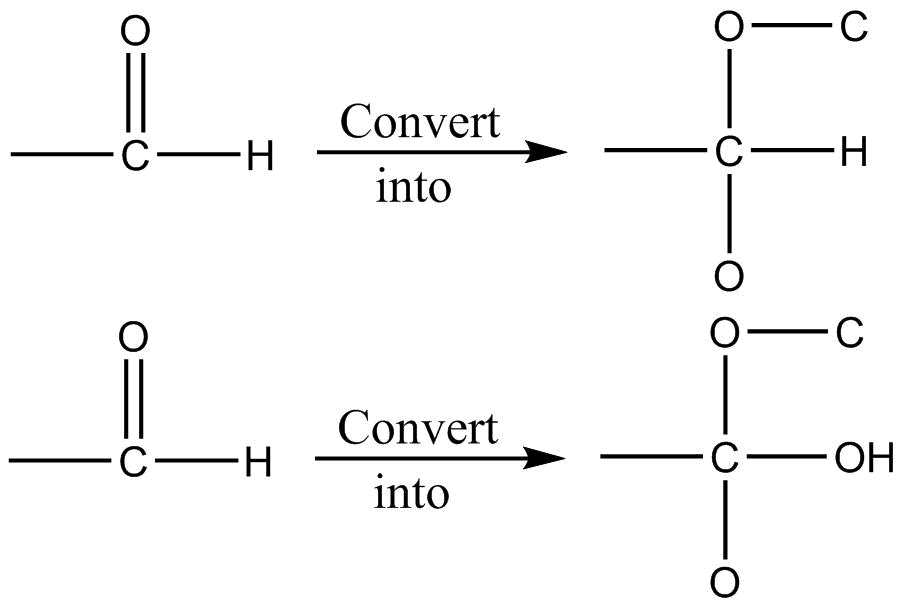
Break multiple bonds and add one more same atom to that. After this apply Rule1 and Rule 2.
So, the answer is −OH>COOH>−CHO>−CH2OH
This is the decreasing priority order.
Multiple bonds are treated as separate single bonds.
Note: −CH=O is given priority over −CH2OH
I>Br>Cl
OH>NO2>NH2>COOH>CHO>CH2
In geometric isomers:
Z−isomer > E−isomer
