Question
Question: Identify the correct $K_b$ order in the following compounds : ...
Identify the correct Kb order in the following compounds :
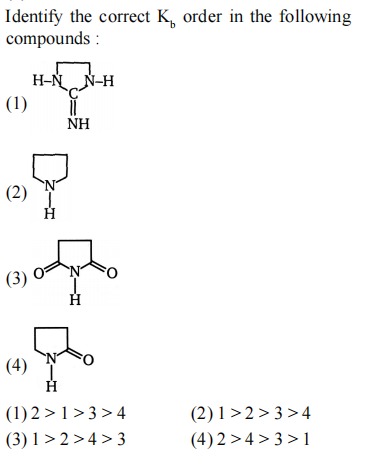
A
2>1>3>4
B
1>2>3>4
C
1>2>4>3
D
2>4>3>1
Answer
1 > 2 > 4 > 3
Explanation
Solution
Explanation:
- (1) is an imidazole derivative where one nitrogen is pyridine‐like (available lone pair), making it quite basic.
- (2) is pyrrole; its lone pair is part of the aromatic sextet so it’s less basic than (1).
- (4) is a lactam type (one C=O group) with the nitrogen lone pair partly delocalized into the carbonyl, making it less basic than pyrrole.
- (3) with two C=O groups (an imide type) has its lone pair much more delocalized and is the least basic.
Thus, the order of basicity (K₍b₎ order) is:
(1) > (2) > (4) > (3)
