Question
Question: Identify the compounds A, B, C and D. \( {\text{Aniline}}\xrightarrow[{{\text{(b) warm }}{{\text{...
Identify the compounds A, B, C and D.
Aniline(a) NaNO2, HCl 0−5oC(b) warm H2O[A]Zndust[B]Fe/Cl2[C]Nadry[D]
Solution
Aniline has the molecular formula C6H5NH2 . It is the simplest aromatic amine as it has the phenyl group attached to the amino group. The first reaction is diazotization followed by hydrolysis. The second reaction is removal to the hydroxyl group. The third reaction is the addition of chloride. The fourth reaction is Fittig reaction.
Complete Step by step solution:
The reaction of aniline with NaNO2 and HCl is called a diazotization reaction. The compound obtained is called diazonium salt. The general method of preparation of diazonium compounds is the reaction of aromatic amines with sodium nitrate along with an additional acid. The product that is diazonium salt is stable only at 0−5∘ C. The product that is diazonium salt on reaction with warm water forms phenol.
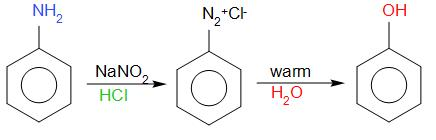
Phenol
The product obtained from the above-mentioned reaction is phenol that is compound A.
Phenol gets converted into phenoxide ion and it releases proton. The release proton accepts an electron from zinc and forms hydrogen radical. The homolytic fission of carbon of the phenyl ring and O− takes place due to heating. Then zinc forms zinc oxide and phenyl radical produces a bond with hydrogen radical. In this way benzene is produced as a product that is compound B.
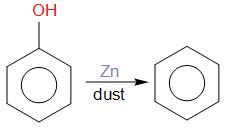
Benzene
Benzene reacts with either chlorine or bromine in the presence of a catalyst. This catalyst can be either aluminium chloride or iron. The reaction takes place at room temperature and one of the hydrogen atoms on the ring is replaced by chlorine or bromine atom.
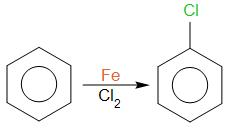
Chlorobenzene
The product obtained is chlorobenzene that is compound C.
Now chlorobenzene reacts with sodium metal in dry ether to give diphenyl. This is known as Fittig reaction.
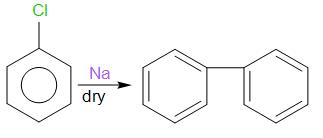
Diphenyl
The product obtained is diphenyl which is compound D.
Note:
In Fittig reaction, any two aryl halides are coupled in presence of sodium metal in dry ether to give biaryl. In the case of aliphatic haloalkanes, this reaction is called wurtz reaction.
