Question
Question: Identify the compound Y in the following reaction: 
(A) 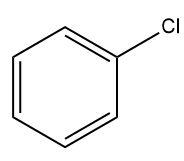
(B) 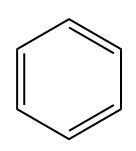
(C) 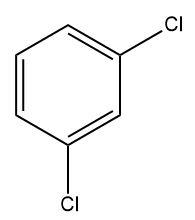
(D) 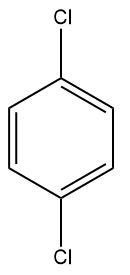
Solution
The reaction that converts aniline into the corresponding diazonium salt that further gets acted upon by copper salts leads to the formation of different benzene derivatives depending upon the anion present in the copper salts.
Complete Step By Step Answer:
The given reaction is known as Sandmeyer’s reaction in which copper salts are utilized as active reagents to obtain aryl chlorides from diazonium salts.
The first step of the reaction is to form a diazonium salt from aniline by the action of sodium nitrite and hydrochloric acid taken at low temperatures. The two reagents combine to give nitrous acid in situ that leads to the formation of diazonium salt.
The benzene diazonium salt further undergoes a radical nucleophilic aromatic substitution reaction in the presence of cuprous chloride that leads to the replacement of the diazonium group by chlorine substituent and a mono chlorinated benzene ring is formed as the final product.
For di-chlorination to take place, the ring needs to be sufficiently activated, which does not happen in the Sandmeyer’s reaction.
Only benzene ring can be obtained as a product by further adding a reagent that removes the chlorine substituent attached to the benzene ring.
⇒ Thus, option (A) is correct and compound Y is chloro benzene.
Note:
The formula of the copper salt given in the equation suggests that it is cuprous chloride. The presence of two chloride ions in the formula of the reagent does not ensure that the product formed will be di-substituted in nature. The benzene ring is not that activated to allow more than one nucleophilic attack.
