Question
Question: Identify the compound C. 
Solution
The t−BuOK is the tert- butoxide which is the conjugate base of tert- butanol. The potassium tert- butoxide helps in catalyzing the reaction of hydrosilanes and heterocyclic compounds which helps in formation of silyl derivatives. The tert- butoxide is a strong base because of the inductive effect and the presence of electron donating methyl groups.
Complete step by step solution:
The methyl groups in tert- butoxide reduces the polarity of hydroxide bond in t- butyl alcohol which makes it a weaker acid. And a strong conjugate base. The reason is salvation also because it is difficult for tert- butoxide to dissociate into ions so that the compound gets destabilised into ethoxide. It is a weak nucleophile because of the presence of steric hindrance. So when the above organic compound reacts with bromoform and tert- butoxide the formation of the following product occurs.
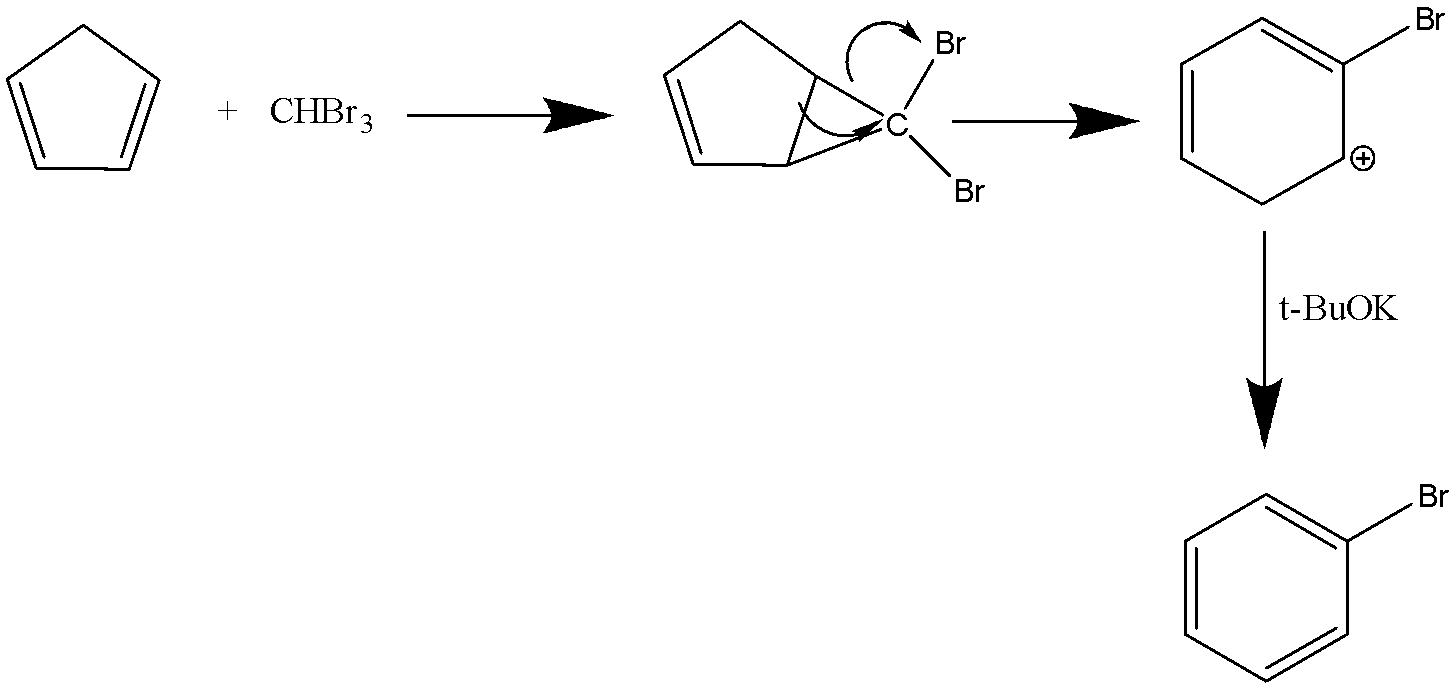
The product formed after the reaction is bromobenzene .In the above reaction Haloform reaction has occurred. In this mechanism the disproportion of the halogen with presence of hydroxide ion occurs. The reaction is of the nucleophilic substitution reaction. The tert- butoxide exists as a tetrameric cubane type cluster. It is a colourless solid which is widely used in the formation of organic compounds.
Hence, the compound C is bromobenzene ( C6H5Br ).
Note:
The tert butoxide is a non nucleophilic strong base. Due to its steric hindrance by the methyl groups it can participate in nucleophilic reaction. The compound formed in the reaction is complex in nature which affects the reactivity of reagent. Schlosser’s base is a mixture of the alkoxide and alkyl lithium compound, and is a related but stronger base.
