Question
Question: Identify the chiral molecule among the following pair? 
Solution
A chiral molecule is the atom which is attached to four different groups or functional groups. It is also known as an asymmetric carbon atom. According to Le-Bel-van't-Hoff rule, the number of stereoisomers of an organic compound can be calculated as2n, where n is the number of chiral or asymmetric carbon atoms.
Complete answer:
Since, now we have a basic knowledge about chiral atoms. So let’s proceed towards our given option.
Looking at our first option i.e. 2−chlorobutane – In this molecule as we can see , the carbon atom at which chlorine is attached (marked with red box ) is a asymmetric atom , as it has four different functional group attached to it i.e. H,CH2,CH3,Cl.
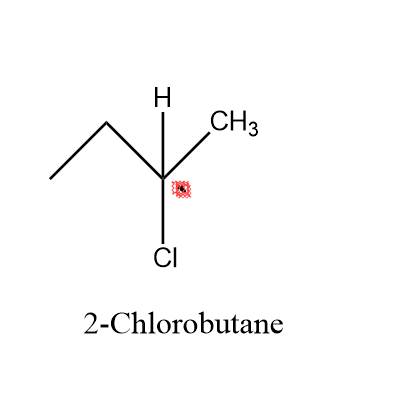
As , we can see clearly in the figure that our second carbon is an asymmetric atom .
Hence, we can say that our 2−chlorobutane is a chiral molecule.
Coming to our next option 1− chlorobutane, it is an achiral molecule, as it does not have any asymmetric carbon atom. Each atom is attached to two similar functional groups .
Note:
Phenomenon chirality is a very important concept especially in biology because most of the biological molecules like amino acids, carbohydrates- sugars, starch , cellulose , are chiral in nature and these molecules are the building block for larger molecules like nucleic acid, proteins which is very important for our body composition. All amino acids except glycine are chiral , because it does not contain any chiral centre . Also Chirality is introduced by “Sir William Thomsan” in 1894.
