Question
Question: Identify the binary mixtures that can be separated into individual compounds, by differential extrac...
Identify the binary mixtures that can be separated into individual compounds, by differential extraction, as shown in the given scheme:
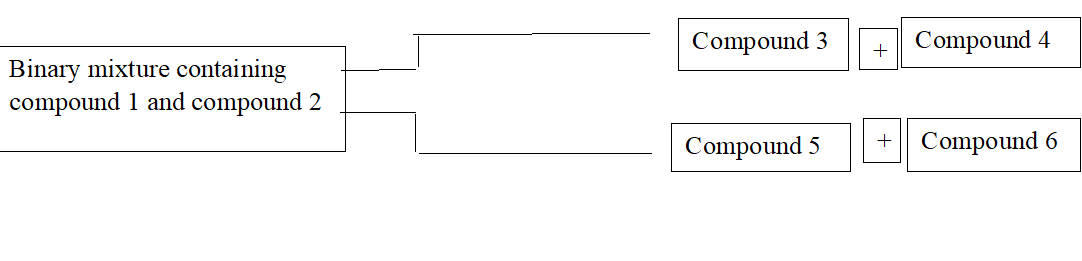
This question has multiple correct options
A. C6H5OH,C6H5COOH
B. C6H5COOH,C6H5CH2OH
C. C6H5CH2OH,C6H5OH
D. C6H5CH2OH,C6H5CH2COOH
Solution
Binary mixtures are those which have two components. In this only one component of the mixture is collected in pure form.
Complete Solution :
Binary mixtures have usually two components out of which both can be liquid, one can be solid and solid etc. Binary mixtures which contain solid and liquid can be separated by physical methods. Others can be separated by some other processes like distillation, neutralization, centrifugation etc. the separation is based on the solubility of acidic/basic solution. In this type of separation only one component of mixture is collected which is pure in nature.
In the question if we take option
A. C6H5OH,C6H5COOH can be separated with NaHCO3 but with NaOHis not possible this case, so option A is not correct.
B. C6H5COOH,C6H5CH2OH separation is possible by both NaHCO3and NaOH in this case.
C. C6H5CH2OH,C6H5OH can be separated with NaOH only not with NaHCO3 so this option is not correct.
D. C6H5CH2OH,C6H5CH2COOH separation is possible by both NaHCO3 and NaOH in this case.
So, the correct answer is “Option B and D”.
Note: Separation of binary compounds are depend upon the strength of its acidity and basicity and strength of some organic compounds can be show by:
C6H5COOH,C6H5CH2COOH>H2CO3>C6H5OH>H2O>C6H5CH2OH
