Question
Question: Identify the alkyne in the following sequence of reactions, $\text{Alkyne} \xrightarrow[\text{Lin...
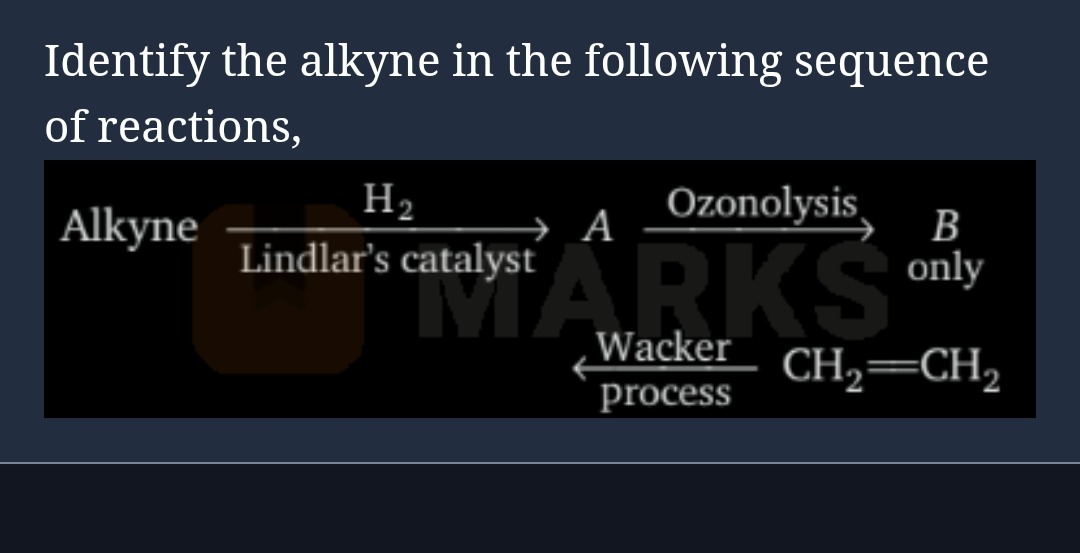
Identify the alkyne in the following sequence of reactions,
AlkyneH2Lindlar’s catalystAOzonolysisB only
CH2=CH2Wackerprocess
But-2-yne
Solution
The sequence of reactions is:
Alkyne H2Lindlar’s catalystAOzonolysisB only
Hydrogenation of an alkyne with Lindlar's catalyst produces a cis-alkene. Let the alkyne be R1-C≡C-R2. Then A is cis-R1-CH=CH-R2.
Ozonolysis of alkene A produces carbonyl compounds by cleaving the double bond. If the ozonolysis produces only one compound B, then the alkene A must be symmetrical. A symmetrical alkene is of the form R-CH=CH-R or R2C=CR2. Since A is formed by hydrogenation of an alkyne with Lindlar's catalyst, A must be of the form R-CH=CH-R. Therefore, the alkyne must be symmetrical, R-C≡C-R.
Ozonolysis of R-CH=CH-R gives 2 molecules of R-CHO. So, B is R-CHO. The condition "B only" means that only one type of carbonyl compound is formed, which is consistent with A being a symmetrical alkene and thus the alkyne being symmetrical.
Now let's consider the last part of the diagram: CH2=CH2Wackerprocess. This indicates that ethene is formed from B through a process related to the Wacker process. The Wacker process converts ethene to acetaldehyde (CH3CHO). The reverse reaction is not the Wacker process. However, the diagram might be hinting at the identity of B.
Let's assume that B is acetaldehyde (CH3CHO). If B is acetaldehyde, then R in R-CHO is CH3. The alkyne R-C≡C-R is CH3-C≡C-CH3 (but-2-yne).
Let's check this sequence:
Alkyne: But-2-yne (CH3-C≡C-CH3)
H2Lindlar’s catalyst A: cis-But-2-ene (CH3-CH=CH-CH3)
Ozonolysis B: Ozonolysis of cis-but-2-ene cleaves the double bond and forms two molecules of acetaldehyde (CH3CHO). So, B is acetaldehyde. This fits the condition that only B is formed.
Now, let's consider the last part. If B is acetaldehyde, the diagram shows CH2=CH2Wackerprocess. This means acetaldehyde is converted to ethene through a process related to the Wacker process. While the Wacker process is ethene to acetaldehyde, perhaps the diagram intends to show a connection between acetaldehyde and ethene. In the context of typical organic reactions, it is possible to convert acetaldehyde to ethene, for example, by reduction to ethanol followed by dehydration. However, the mention of the Wacker process is specific.
Let's reconsider the possibility that the diagram is implying that B is the product of the Wacker process, which is acetaldehyde, formed from ethene. So, perhaps the diagram is trying to say that B is acetaldehyde, which is obtained from ethene by the Wacker process.
Based on the initial reaction sequence and the condition that only one product B is formed from ozonolysis, the alkyne must be symmetrical. If the alkyne is but-2-yne, then A is cis-but-2-ene, and B is acetaldehyde.
