Question
Question: Identify the A. 
(A)
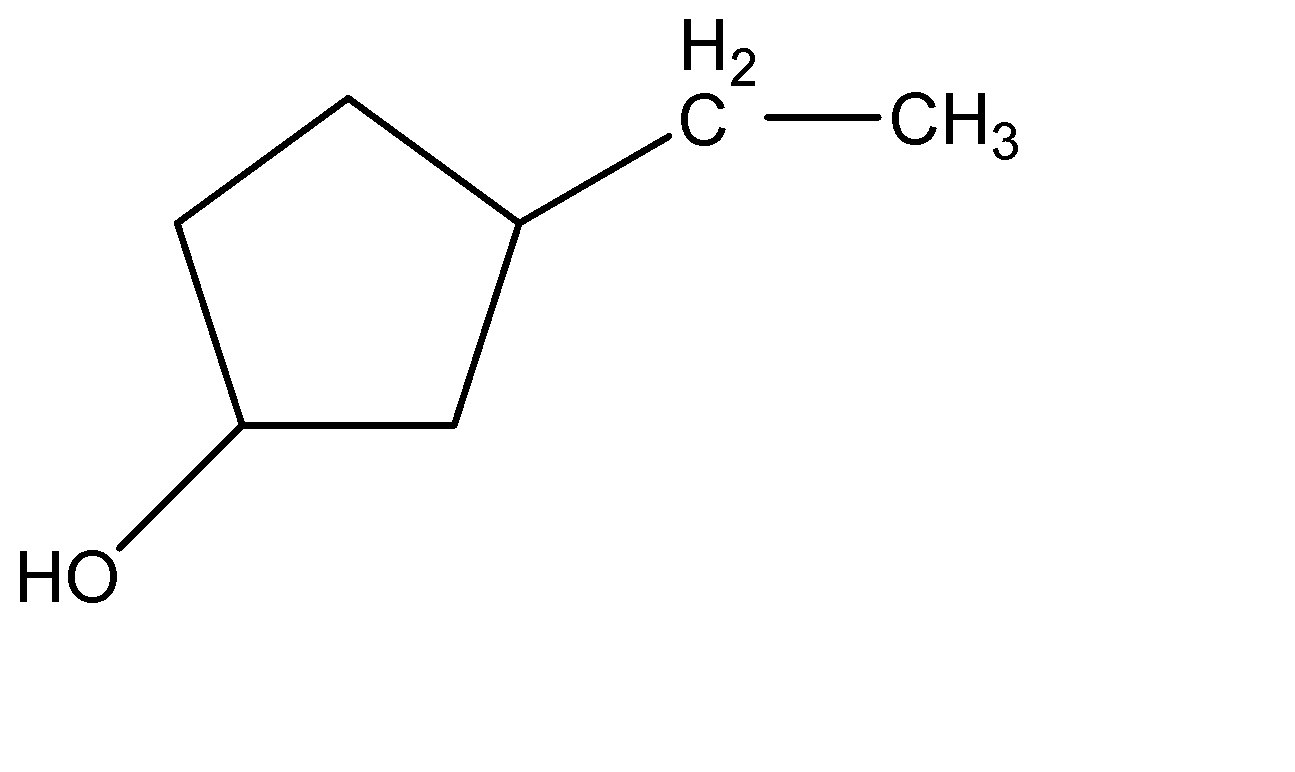
(B)
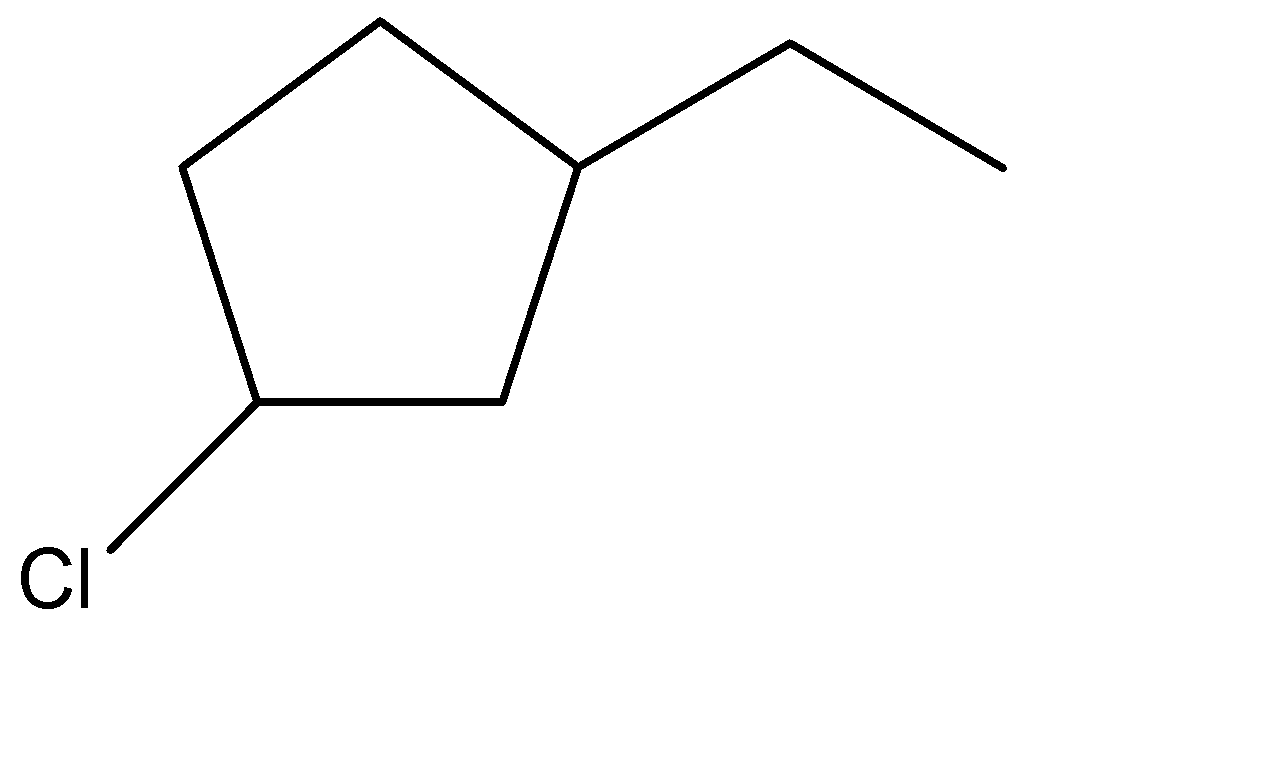
(C)
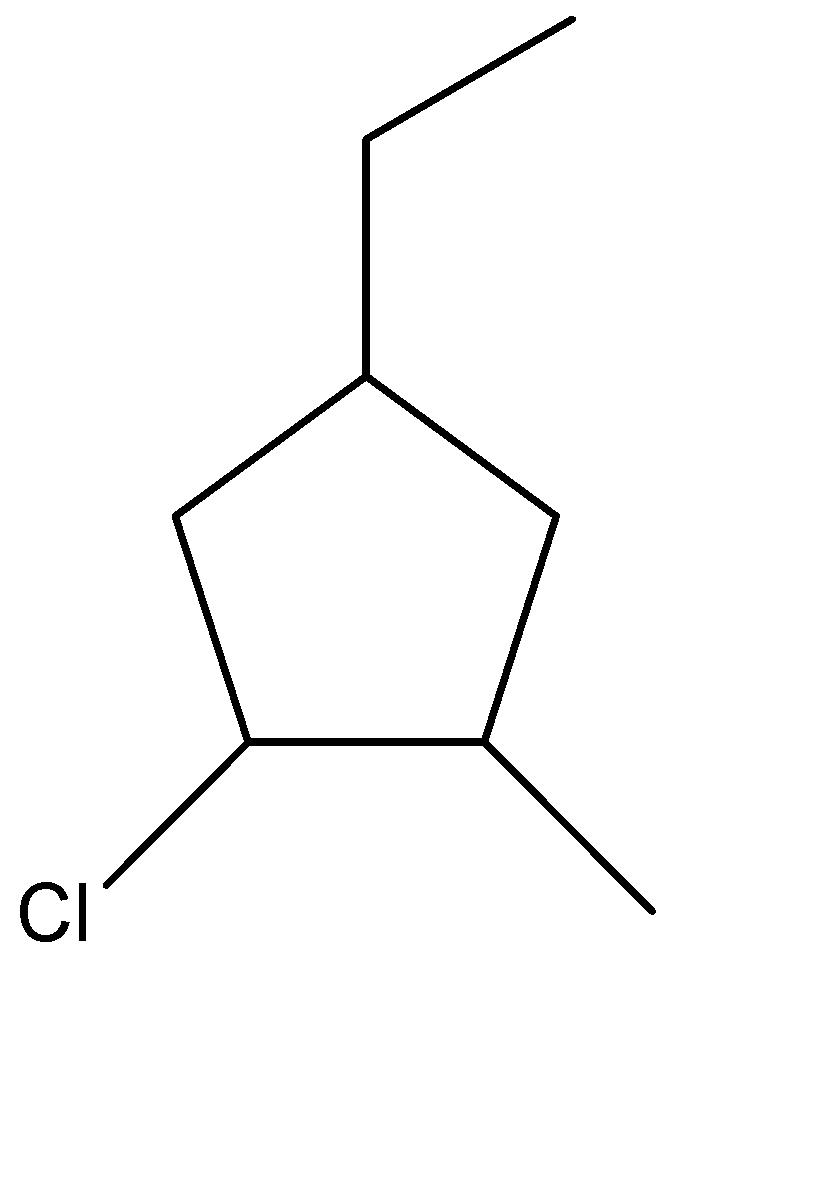
(D)
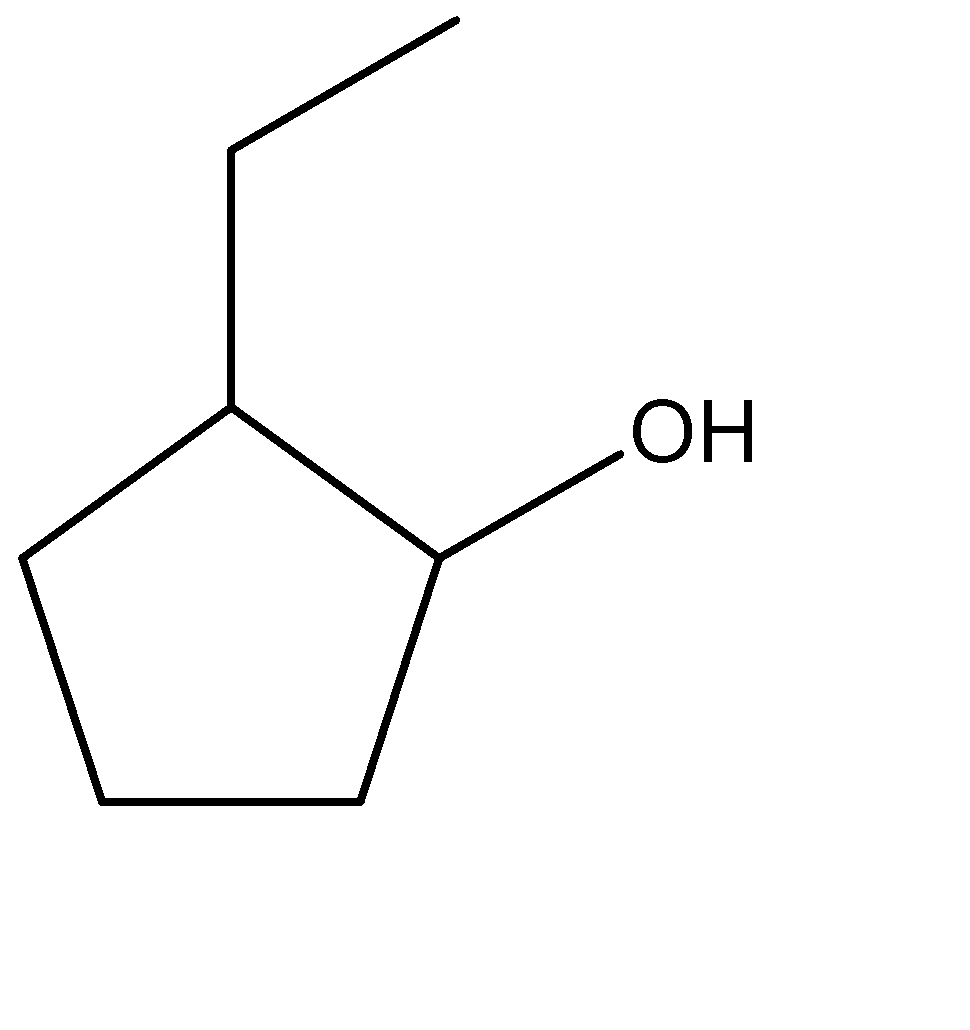
Solution
As we can see that the reagents given in the reaction refers to the Clemmenson reduction, so we will perform Clemmenson reduction on the given reactant. Since the reactant has an acid sensitive group i.e., alcohol group hence will react with hydrochloric acid as well.
Complete answer:
Let us first discuss about Clemmenson reduction followed by the steps of this reaction:-
-Clemmensen Reduction: It is a chemical reaction in organic chemistry where reduction of ketones or aldehydes to alkanes takes place using zinc amalgam and concentrated hydrochloric acid. The substrate used in this reaction must be tolerant to strong acidic conditions.
The reaction takes places as follows:-
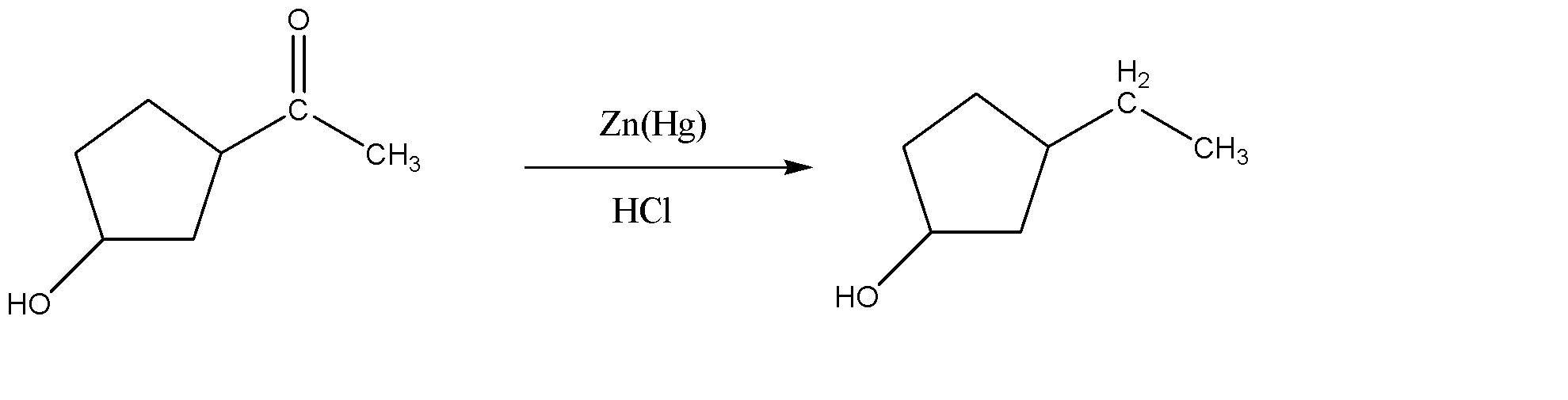
-As we can see, the reactant has a carbonyl group (ketone), so it can easily be reduced to alkane as shown above.
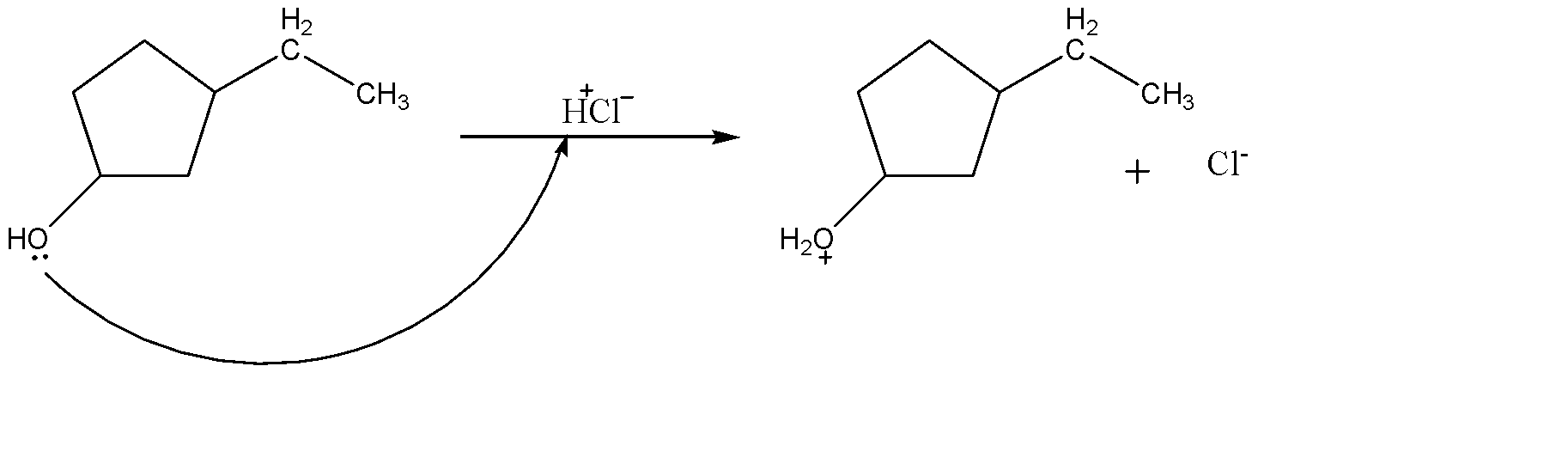
-Now after the formation of alkane group, the reactant also has an alcohol group which is sensitive to the strong acid due to which the electron cloud on oxygen tend to act as nucleophile and attack on the H+ion of hydrochloric acid.
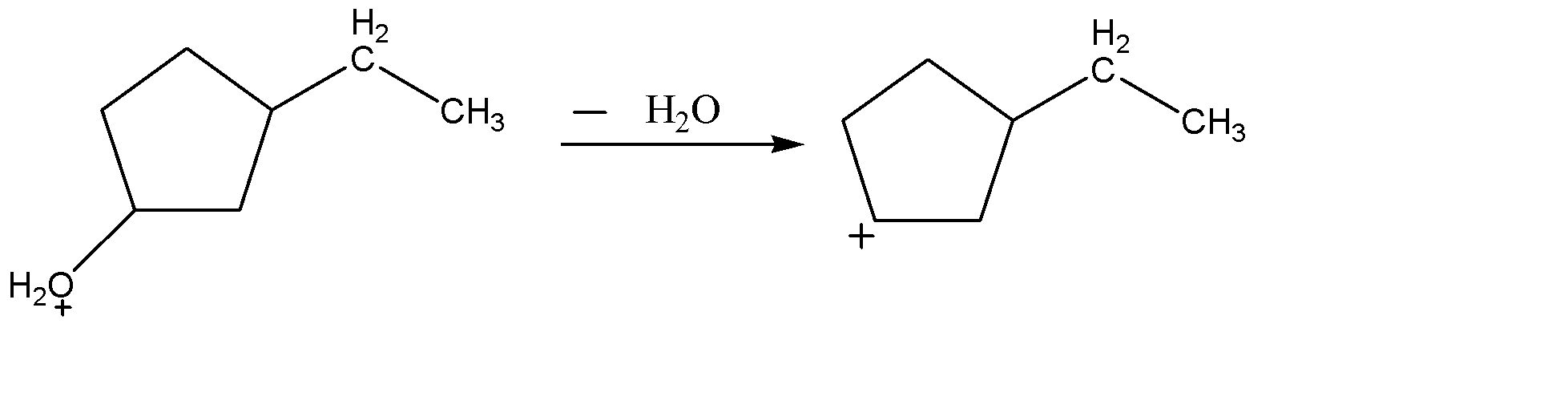
-As oxygen atoms are highly electronegative in nature, so it cannot bear negative charge on itself and will tell you to get free of it by taking the electron density of C-O bond and releasing itself as a water molecule. This leads to the formation of carbocation.
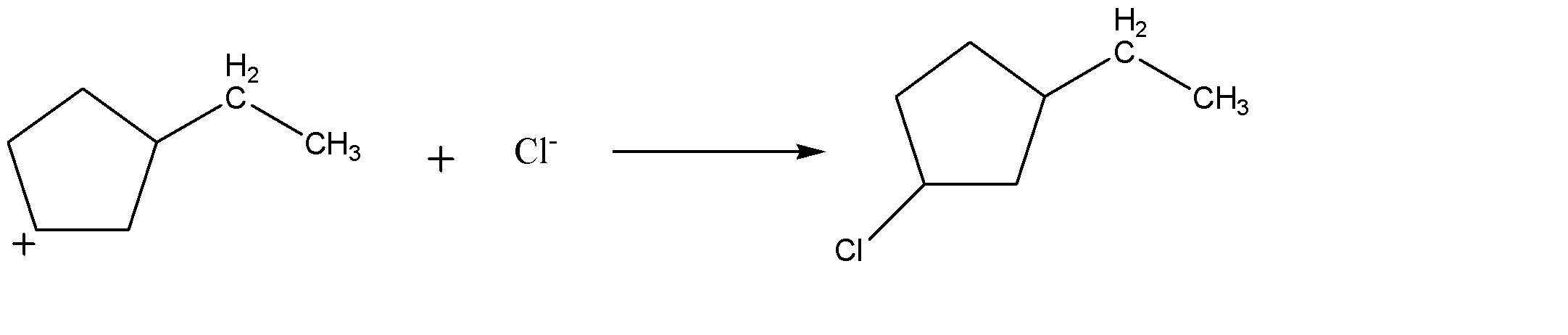
-This carbocation acts as an electrophile and chlorine ion attacks it and forms a sigma bond with the molecule as shown above.
Hence, the correct option is: (B) .
Note:
-Remember that never use acid sensitive reactants in case of Clemmenson reduction, instead make them reduced with the help of Wolf-Kishner reduction which is ran under strongly basic conditions.
-In Clemmenson reduction, the molecules get reduced on the surface of Zinc metal.
