Question
Question: Identify the A and B in the following reaction: \(C{H_3} - CH = C{H_2}\xrightarrow{{HBr}}A\xrighta...
Identify the A and B in the following reaction:
CH3−CH=CH2HBrAKOH aclB
Solution
Markovnikov rule states that in addition of protic acid (HX) to an unsymmetrical alkene, acidic hydrogen gets attached to that carbon atom of double bond which has more hydrogen atoms and halide group gets attached to that carbon atom of double bond which is attached with more alkene groups.
Complete step by step answer:
Reaction given in this question is CH3−CH=CH2HBrAKOH aclB and we have to identify A and B in this reaction.
In the first part of the reaction, that is, CH3−CH=CH2HBrA anti markovnikov rule will be followed. Markonikov rule states that in addition of protic acid (HX) to an unsymmetrical alkene, acidic hydrogen gets attached to that carbon atom of double bond which has more hydrogen atoms and halide group (X) gets attached to that carbon atom of double bond which is attached with more alkene groups. In this reactant, the carbon atom of CH2 has more hydrogen atoms as compared to CH. So, according to anti markovnikov rule hydrogen atom will be attached to CH2 group and bromine atom will be attached to CH group. So, the resulting compound that is A will be:
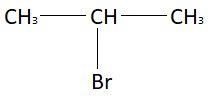
The chemical formula of this compound is C3H7Br.
So the first step of reaction is:
CH3−CH=CH2HBrCH3−CHBr−CH3
Second step of reaction is:
CH3−CHBr−CH3KOH aclB
In this reaction halogen of the reactant will be replaced with OH group of KOH and KBr will be released. So, B is C3H7OH and the complete reaction is:
CH3−CH=CH2HBrCH3−CHBr−CH3KOH aclCH3−CHOH−CH3
Hence A and B are C3H7Br and C3H7OH respectively.
Note:
Anti-markovnikov rule is opposite to the markovnikov rule. According to Anti-markovnikov rule hydrogen atom of HX gets attached to that carbon atom of double bonded carbon which has less number of hydrogen atoms and halogen gets attached to that carbon atom of double bonded carbon atom which has more number of hydrogen atoms.
