Question
Question: Identify tertiary alkyl halide : (A) Tert-butyl bromide (B) 2-methyl-2-bromopropane (C) Both A...
Identify tertiary alkyl halide :
(A) Tert-butyl bromide
(B) 2-methyl-2-bromopropane
(C) Both A and B
(D) None of these
Solution
Write the expanded structure of the organic compounds mentioned above. Understand the meaning of primary, secondary and tertiary carbon atoms. The group attached to it will also get the name. E.g. A halide atom attached to tertiary carbon will become tertiary alkyl halide. The classification of alkyl halides mentioned above is done based on the number of alkyl groups directly attached to the carbon atom under consideration.
Complete step by step solution:
The number of carbon atoms or groups attached to the carbon atom under consideration will help to determine the degree of a carbon atom. Primary carbon has 1 carbon atom or group attached to it. Similarly, secondary and tertiary carbon atoms have 2 and 3 carbon atoms or groups attached respectively. We will now draw the expanded structure of the organic compounds given in the options above as suggested in the hint.
(A) Tert-butyl bromide
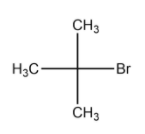
In the above compound, the carbon atom to which the halogen atom is attached to 3 carbon groups(in this case, a methyl group). Hence the above compound is a tertiary alkyl halide.
(B)2 -methyl-2-bromopropane
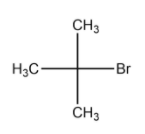
In the above compound, the carbon atom to which the halogen atom is attached to 3 carbon groups(in this case, a methyl group). Hence the above compound is a tertiary alkyl halide.
Therefore, the correct answer is option (C).
Note: The compounds (A) and (B) both have the same expanded structure. This is because tert-butyl bromide is the common name for compounds however the IUPAC compound of the same compound is 2 -methyl-2-bromopropane.
