Question
Question: Identify R and predict the type of reaction 
(A) 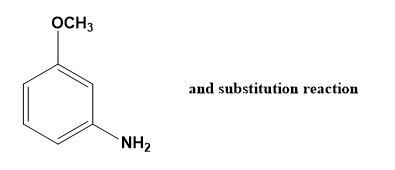
(B) 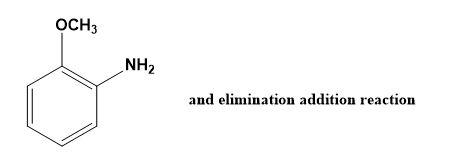
(C) 
(D) 
Solution
Here as the reaction is carried out in presence of NaNH2, the halide group in the molecule would be simply replaced by the electron donating group. By this we can concentrate on one option only but, this reaction gives two products.
Complete step by step solution:
Let us discuss the given reaction directly, It is given as when m-bromo anisole reacts with NaNH2, the product formed will be- The mechanism for the above given reaction is,
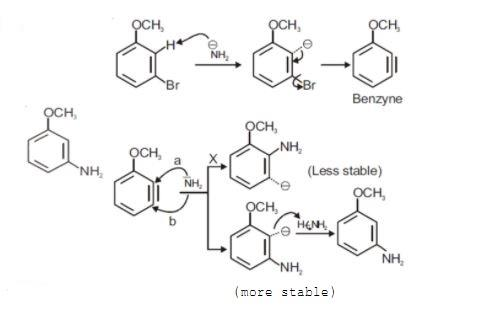
Here, we can say that,
-The bromide ion is substituted at the meta position of the benzene ring. Thus, when an electron donating group attacks the molecule it would primarily be at meta position replacing the -Br ion with −NH2 ion.
-But stability is defined when the electron withdrawing group is close to the negative charge. Thus, −NH2 ion has two sites where it can attack and form the resulting molecule which would be stable.
-So, −NH2 ion attacks on meta and ortho positions. When −NH2 attacks at ortho position, the negative charge is at a meta position which is quite unstable. Whereas, when −NH2 attacks at a meta position, the negative charge is at an ortho position which is firmly stable. Thus, we consider the more stable molecule which is in fact the required product i.e. m- amino anisole. Therefore, we can say that m- bromo anisole reacts with NaNH2 to give m- amino anisole by substitution reaction which goes by addition-elimination pathway.
Hence, option (A) is correct.
Note: Do note that o- amino anisole is also formed while the reaction takes place i.e. option (B) but due to its instability we do not consider the same. Also, option (C) and (D) would never be the answer as the products mentioned can never be produced by cine substitution.
