Question
Question: Identify product and name of reaction. 
(a) 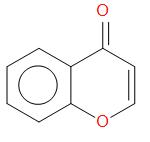 , Aldol condensation
, Aldol condensation
(b) 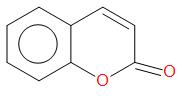 , Perkin reaction
, Perkin reaction
Solution
The reactant in the question is 2-hydroxybenzaldehyde. The common name of 2-hydroxybenzaldehyde is salicylaldehyde. The product formed is an α,β -unsaturated aromatic acid.
Complete Step by step solution:
2-hydroxybenzaldehyde is an organic compound which is colourless, oily liquid and has a bitter almond odour at high concentration. Some of the very commercially important chelating ligands have salicylaldehyde as their precursor. 2-hydroxybenzaldehyde is prepared from phenol and chloroform. This reaction takes place by heating the reactants in the presence of sodium hydroxide or potassium hydroxide. It is called the Reimer-Tiemann reaction. It can also be prepared by condensation of phenol or its derivatives with formaldehyde to give hydroxybenzyl alcohol which is oxidized to aldehyde.
Reaction in which enol or enolate ion reacts with carbonyl compounds to form β -hydroxy aldehyde or β -hydroxy ketone, is called aldol condensation. This reaction is followed by dehydration to give a conjugated enone. It is a good method to form carbon-carbon bonds and an important reaction in organic synthesis. In aldol condensation, there is self-aldol condensation and crossed aldol condensation. In self aldol condensation, both the reactants are same and in crossed aldol condensation, the reactants are two dissimilar carbonyl compounds.
The Perkin reaction is an organic reaction developed by English chemist William Henry Perkin. Perkin reaction is mainly used to make cinnamic acids. Perkin reaction gives an α,β -unsaturated aromatic acid by aldol condensation of aromatic aldehyde and acid anhydride. The base catalyst acting is alkali-salts.
The reaction that takes place is as follows,
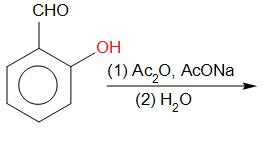
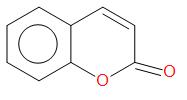
Ortho-hydroxybenzaldehyde reacts with sodium salt and anhydride of carboxylic acid which is usually acetic acid. The product obtained is Coumarin.
Therefore, the correct answer is option (B).
Note:
The other two isomers of hydroxybenzaldehyde are 3-hydroxybenzaldehyde and 4-hydroxybenzaldehyde. Coumarin has a sweet odour and it is a colourless crystalline solid with bitter taste. In plants it serves as a chemical defence against predators.
