Question
Question: Identify correct order of stability:...
Identify correct order of stability:
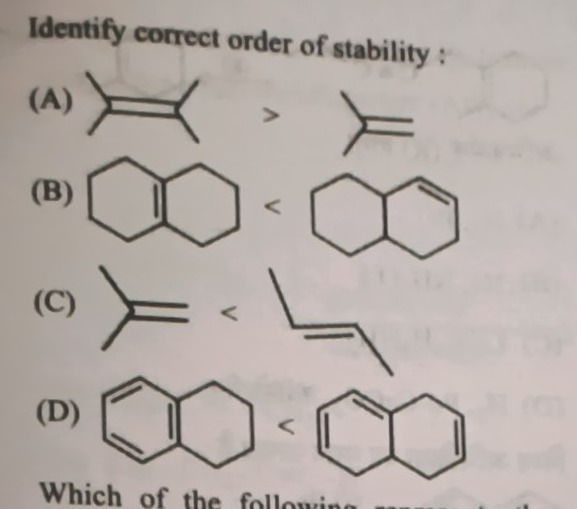
A
2,3-dimethylbut-2-ene > 2-methylpropene
B
Left molecule < Right molecule
C
2-methylpropene < but-2-ene
D
Tetralin < Right molecule
Answer
Options (A) and (B) are correct.
Explanation
Solution
- Stability of alkenes increases with the number of alkyl substituents and hyperconjugation (alpha hydrogens).
- Aromaticity confers significant stability.
- (A) Tetrasubstituted alkene (2,3-dimethylbut-2-ene) is more stable than a trisubstituted alkene (2-methylpropene).
- (B) Comparing isomers of octahydronaphthalene with a double bond, the one with more alpha hydrogens (6 vs 4) is more stable.
- (C) Trisubstituted alkene (2-methylpropene) is more stable than a disubstituted alkene (but-2-ene).
- (D) Aromatic tetralin is much more stable than a non-aromatic bicyclic system.
