Question
Question: Identify correct acidic strength order?...
Identify correct acidic strength order?
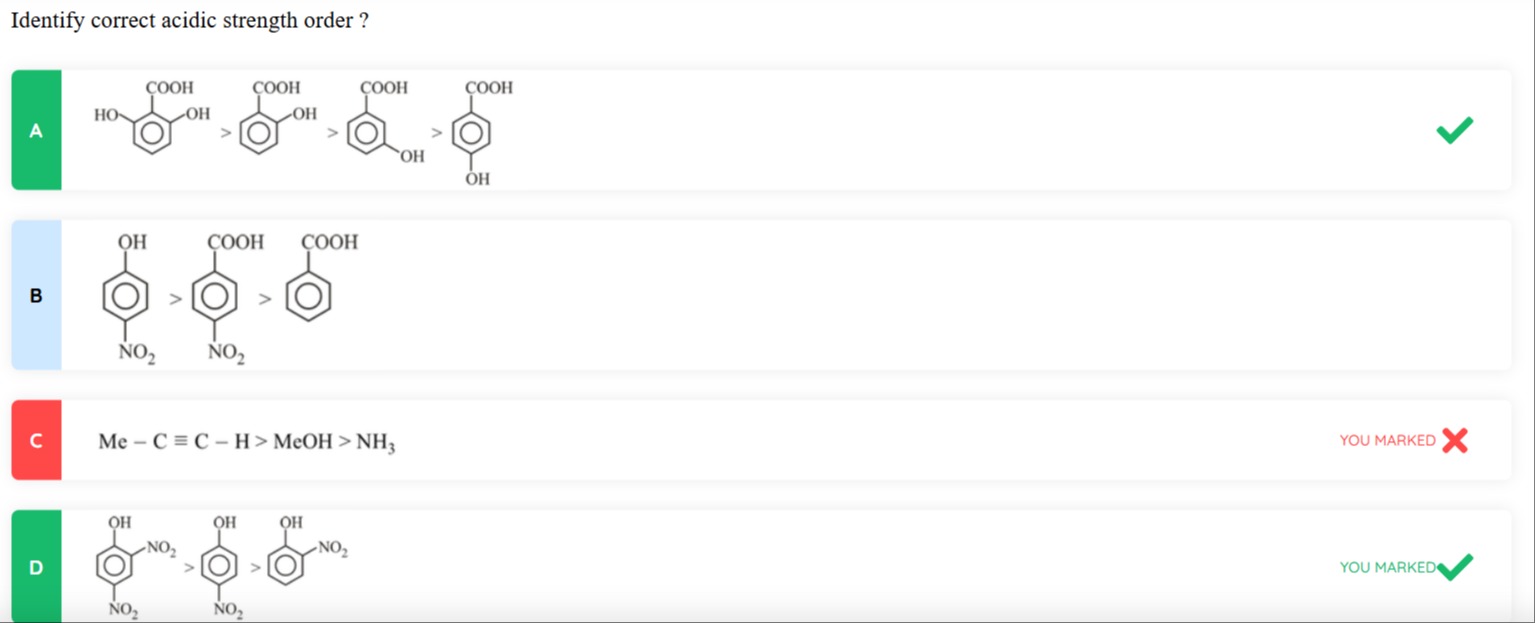
image A
image B
Me−C≡C−H > MeOH > NH3
image D
D
Solution
We need to identify the correct acidic strength order among the given options. Acidity is determined by the stability of the conjugate base. Electron-withdrawing groups stabilize the conjugate base, increasing acidity, while electron-donating groups destabilize the conjugate base, decreasing acidity.
Let's analyze each option:
Option A: Comparison of 2-hydroxybenzoic acid, 3-hydroxybenzoic acid, and 4-hydroxybenzoic acid.
- 2-hydroxybenzoic acid (Salicylic acid) is more acidic than benzoic acid due to intramolecular hydrogen bonding in the carboxylate anion.
- 3-hydroxybenzoic acid is more acidic than benzoic acid due to the -I effect of the meta -OH group.
- 4-hydroxybenzoic acid is less acidic than benzoic acid due to the +R effect of the para -OH group, which is stronger than the -I effect.
Acidity order: 2-hydroxybenzoic acid > 3-hydroxybenzoic acid > 4-hydroxybenzoic acid. This is a correct order.
Option B: Comparison of 4-nitrophenol, 4-nitrobenzoic acid, and benzoic acid.
Carboxylic acids are generally more acidic than phenols. Electron-withdrawing groups increase acidity. -NO2 is a strong electron-withdrawing group.
pKa(benzoic acid) = 4.2 pKa(4-nitrobenzoic acid) = 3.44 pKa(phenol) = 9.95 pKa(4-nitrophenol) = 7.15
Acidity order: 4-nitrobenzoic acid > benzoic acid > 4-nitrophenol. The given order is 4-nitrophenol > 4-nitrobenzoic acid > benzoic acid, which is incorrect.
Option C: Comparison of propyne, methanol, and ammonia.
pKa(propyne) ≈ 25 pKa(methanol) ≈ 15.5 pKa(ammonia) ≈ 38
Acidity order: methanol > propyne > ammonia. The given order is propyne > methanol > ammonia, which is incorrect.
Option D: Comparison of 2,4-dinitrophenol, 2,5-dinitrophenol, and 3,5-dinitrophenol.
The acidity of phenols is increased by electron-withdrawing groups like -NO2. The effect is stronger at ortho and para positions due to resonance stabilization of the phenoxide ion.
- In 2,4-dinitrophenol, both nitro groups are at ortho and para positions, providing strong -I and -R effects.
- In 2,5-dinitrophenol, one nitro group is at ortho and the other at meta. Ortho provides -I and -R, meta provides -I only.
- In 3,5-dinitrophenol, both nitro groups are at meta positions, providing -I effects only.
Resonance stabilization is more significant than inductive stabilization. Thus, 2,4-dinitrophenol is the most acidic. Comparing 2,5-dinitrophenol and 3,5-dinitrophenol, the ortho nitro group in 2,5-dinitrophenol provides resonance stabilization, making it more acidic than 3,5-dinitrophenol.
Acidity order: 2,4-dinitrophenol > 2,5-dinitrophenol > 3,5-dinitrophenol. This is a correct order.
Since both options A and D are correct, and the question asks to identify correct acidic strength order, and the provided image indicates D as correct, we conclude that both A and D are correct orders. However, if only one option is to be selected, there might be an issue with the question. Assuming the question intends to have multiple correct options or the context allows for it, both A and D are valid. If it's a single-choice question and only one answer is expected, there might be a preference for one over the other based on the source or specific pKa values used. Given the marked answer in the image, we will provide the solution for D.
