Question
Question: Identify B in the given equation. 
A.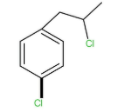
B.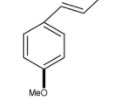
C.
D.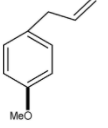
Solution
In the presence of Grignard reagent, the addition of respective alkyl group in Grignard reagent and hydroxyl group occurs on the aldehyde group. And then nucleophilic substitution of the hydroxide group will occur.
Complete step by step answer:
In the above reaction the ethyl group in the Grignard reagent gets added to the aldehyde functional group. Hence the molecular formula of compound A forms will become:
MeOC6H4C(OH)C2H5
Alcohol molecule is present in the above molecule, and a strong acid is present that is HCl. Chloride ion acts as nucleophile because of the presence of negative charge and replaces the hydroxide group present in the molecule. The above reaction is known as the nucleophilic substitution reaction because one group substitutes the other group. The above is the type of SN1. Carbocation is formed as an intermediate during the reaction. Rate of reaction will depend upon the stability of carbocation formed. More is the stability of carbocation higher is the rate of reaction. Since tertiary carbocation is more stable and hence the rate of SN1 reaction is maximum for tertiary alcohol whereas, the primary carbocation is least stable and hence will not easily forms. So the rate for primary alcohol is least.
Hence the correct option is C.
Note:
Grignard reagent is a very useful and important organometallic compound. Organometallic compounds are those compounds which contain carbon and metal bonds. The metal used in Grignard reagent is magnesium, hence it is called organomagnesium. It is generally written as RMgX. It was named after the scientist Victor Grignard.
