Question
Question: Identify A and predict the type of reaction. 
(A)
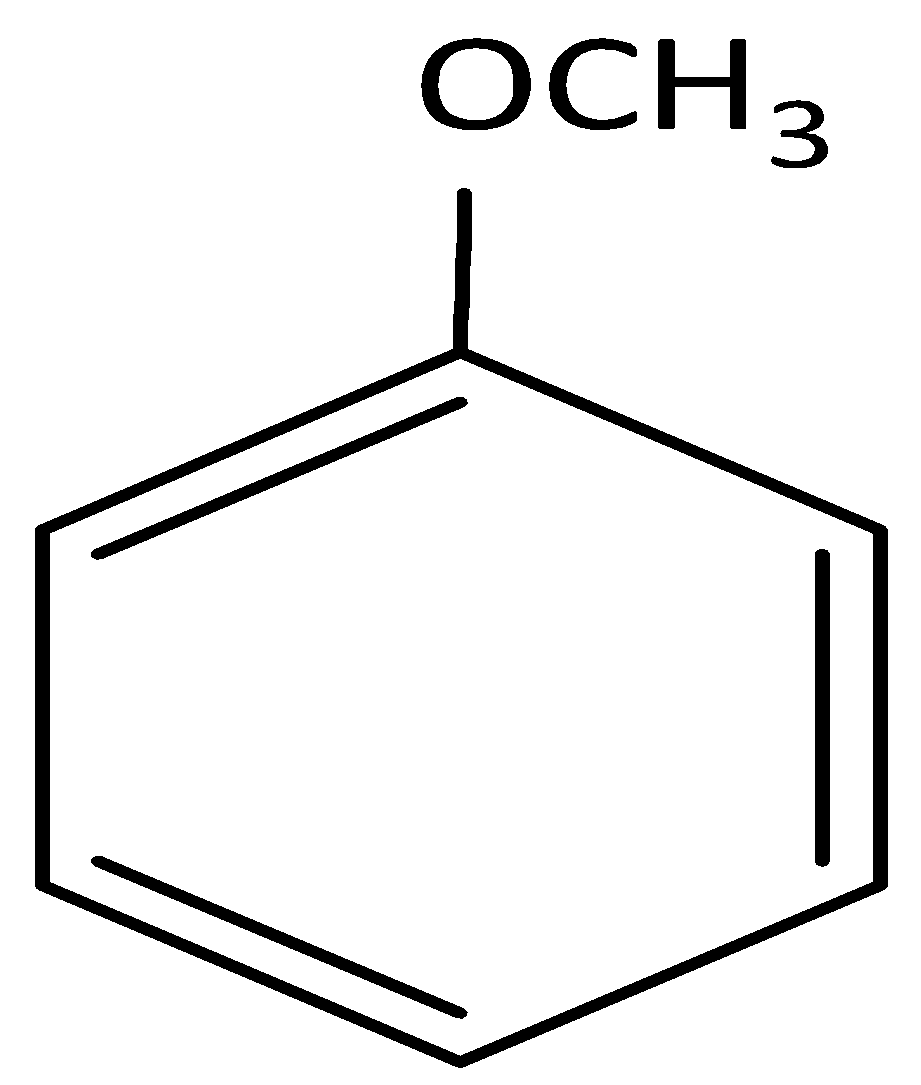 and cine substitution reaction
and cine substitution reaction
(B)
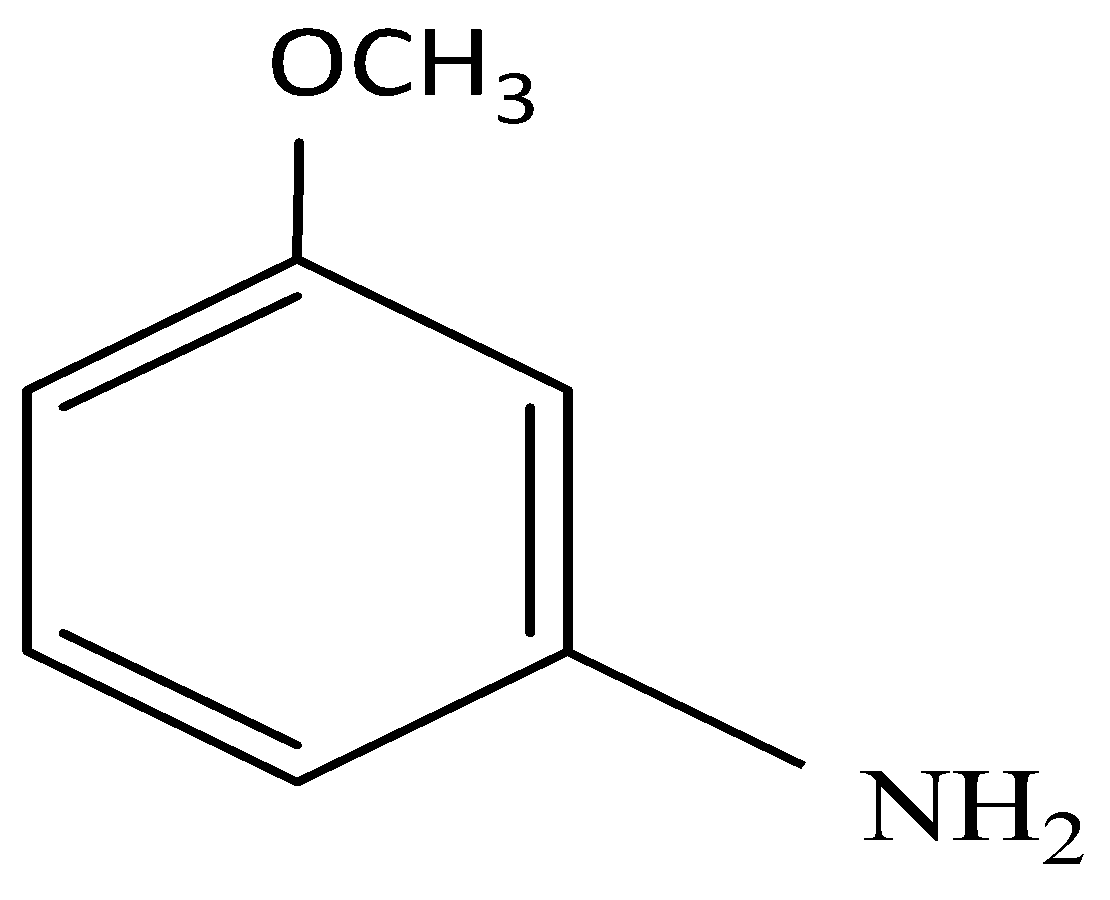 and substitution reaction
and substitution reaction
(C)
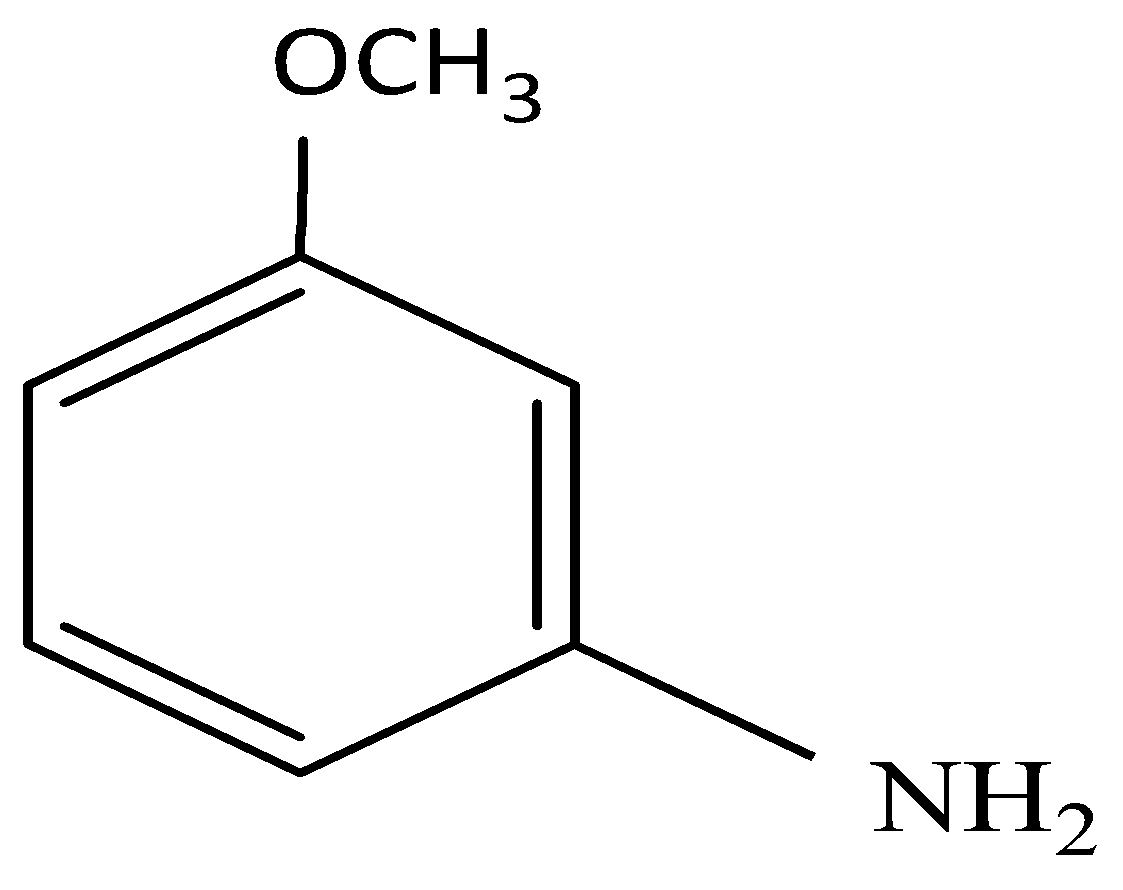 and elimination addition reaction
and elimination addition reaction
(D)
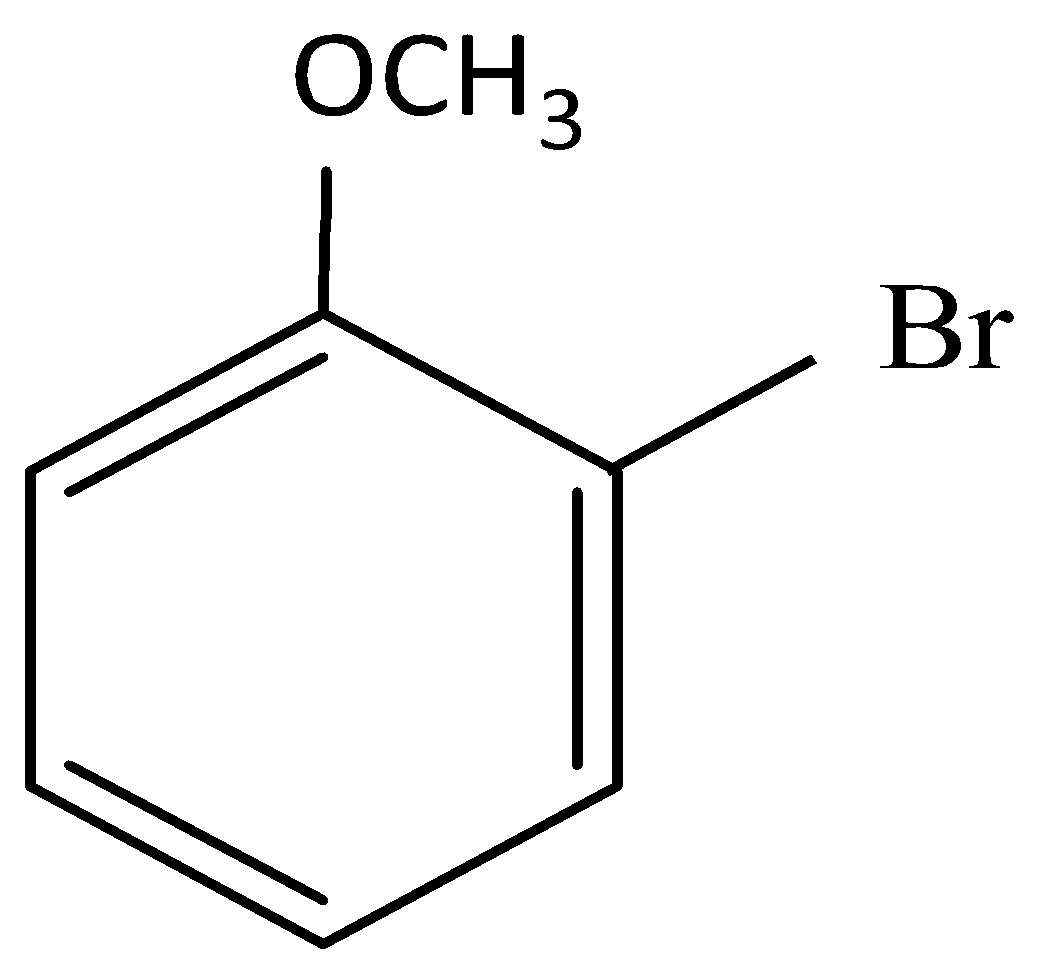 and cine substitution reaction
and cine substitution reaction
Solution
We need to know what is a nucleophile and an electrophile and whether the given compound used in the reaction (NaNH2) is a nucleophile or an electrophile. Electrophile and nucleophile is a chemical species that donate or accept electrons to form a new chemical bond. A nucleophile is usually charged negatively or neutral with a lone couple of donable electrons. Positively loaded or neutral species are called electrophiles that are deficient in electrons and can accept a couple of electrons.
Complete step by step answer:
We have to remember that the NaNH2 is also known as sodium amide or sodamide. It is a strong base capable of deprotonating.
In the given reaction, NaNH2 acts as a nucleophile and the following takes place:
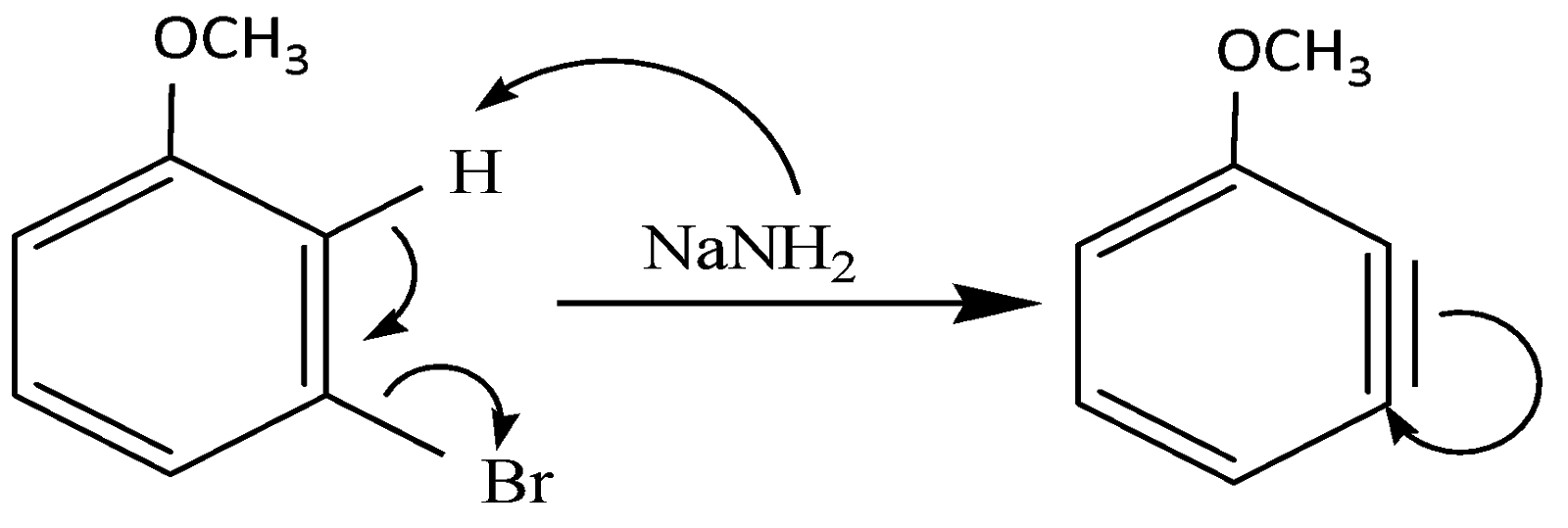
(i) The NH2 deprotonates the H group and as a result it gives its electron to the benzene ring and Br acts as a leaving group and leaves behind a benzyne intermediate. This intermediate is formed as a result of elimination reaction.
(ii) The triple bond breaks and donates its hydrogen to the incoming NH2 group and the resulting molecule is:
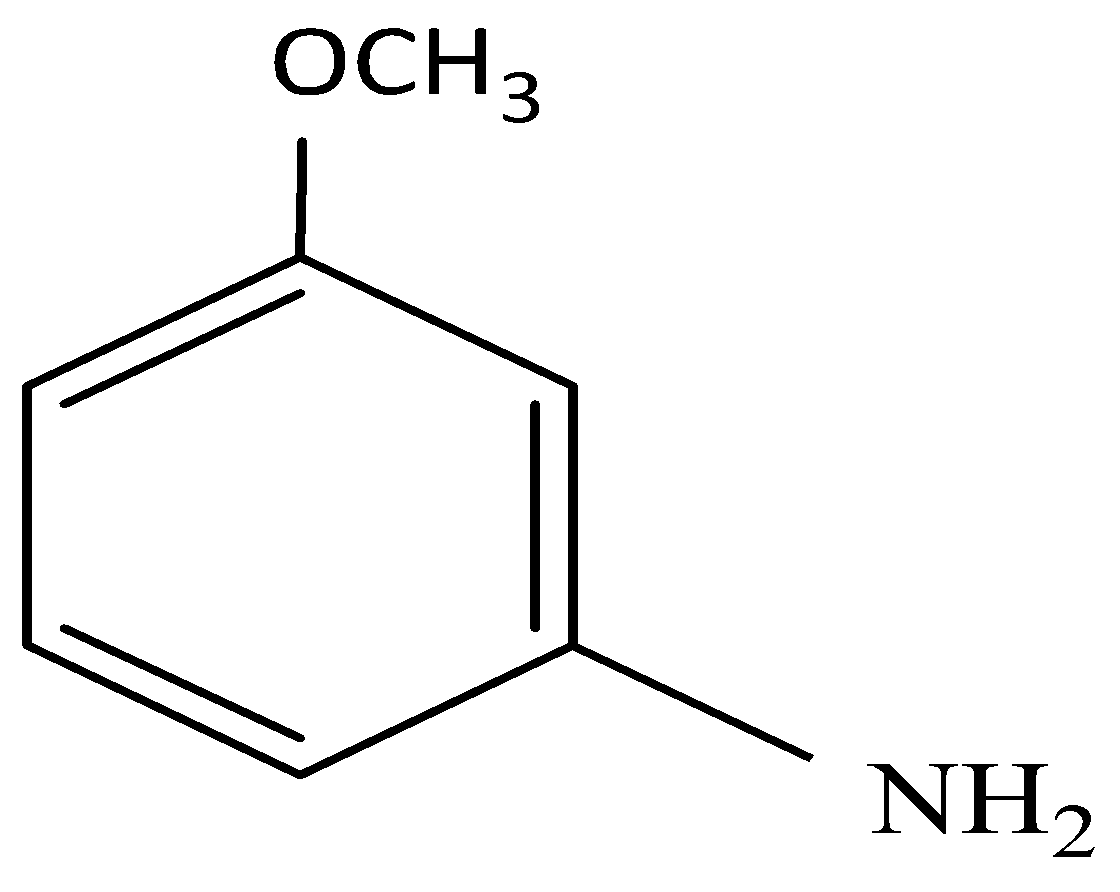
It is clear that elimination of Br is followed by addition of NH2.
So, the correct answer is Option C.
Note: It must be noted that in order to predict the product of such reactions, the nature of the attacking group must be known, i.e. whether the attacking group is a nucleophile or an electrophile. The difference between the two must be clear. Also, nucleophilic elimination addition reaction is also known as nucleophilic aromatic substitution reaction. It is also important to know the difference between a substitution reaction and cine substitution reaction. Cine- substitution is a substitution reaction (generally aromatic) in which the entering group takes up a position adjacent to that occupied by the leaving group.
