Question
Question: Identify A and predict the type of reaction: 
A. 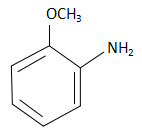 and elimination addition.
and elimination addition.
B. 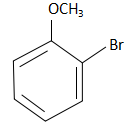 and eine substitution reaction.
and eine substitution reaction.
C.  and substitution reaction.
and substitution reaction.
D. 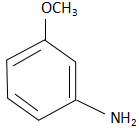 and substitution reaction.
and substitution reaction.
Solution
Sodium amide, commonly called sodamide (systematic name sodium azanide), is the inorganic compound with the formula NaNH2 . It is a salt composed of the sodium cation and the azanide anion. This solid, which is dangerously reactive toward water, is white, but commercial samples are typically gray due to the presence of small quantities of metallic iron from the manufacturing process.
Complete answer:
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions are of prime importance in organic chemistry. Substitution reactions in organic chemistry are classified either as electrophilic or nucleophilic depending upon the reagent involved, whether a reactive intermediate involved in the reaction is a carbocation, a carbanion or a free radical, and whether the substrate is aliphatic or aromatic.
When m-methoxy bromobenzene reacts with sodium azide, the NH2− ions substitute the Br− ions from the reactant and form m-methoxy aniline. The reaction that takes place here is as follows:
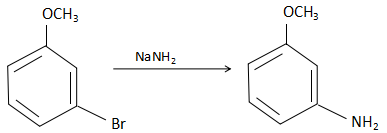
The product in the reactant is m-methoxy aniline and the reaction takes place through a substitution reaction.
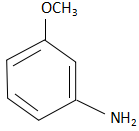
Thus option D is the correct answer.
Note:
NaNH2 conducts electricity in the fused state, its conductance being similar to that of NaOH in a similar state. NaNH2 has been widely employed as a strong base in organic synthesis. NaNH2 is a salt-like material and as such, crystallizes as an infinite polymer. The geometry about sodium is tetrahedral.
