Question
Question: The standard EMF of a cell having one electron change is found to be 0.591 V at 25°CThe equilibrium ...
The standard EMF of a cell having one electron change is found to be 0.591 V at 25°CThe equilibrium constant of the reaction is:
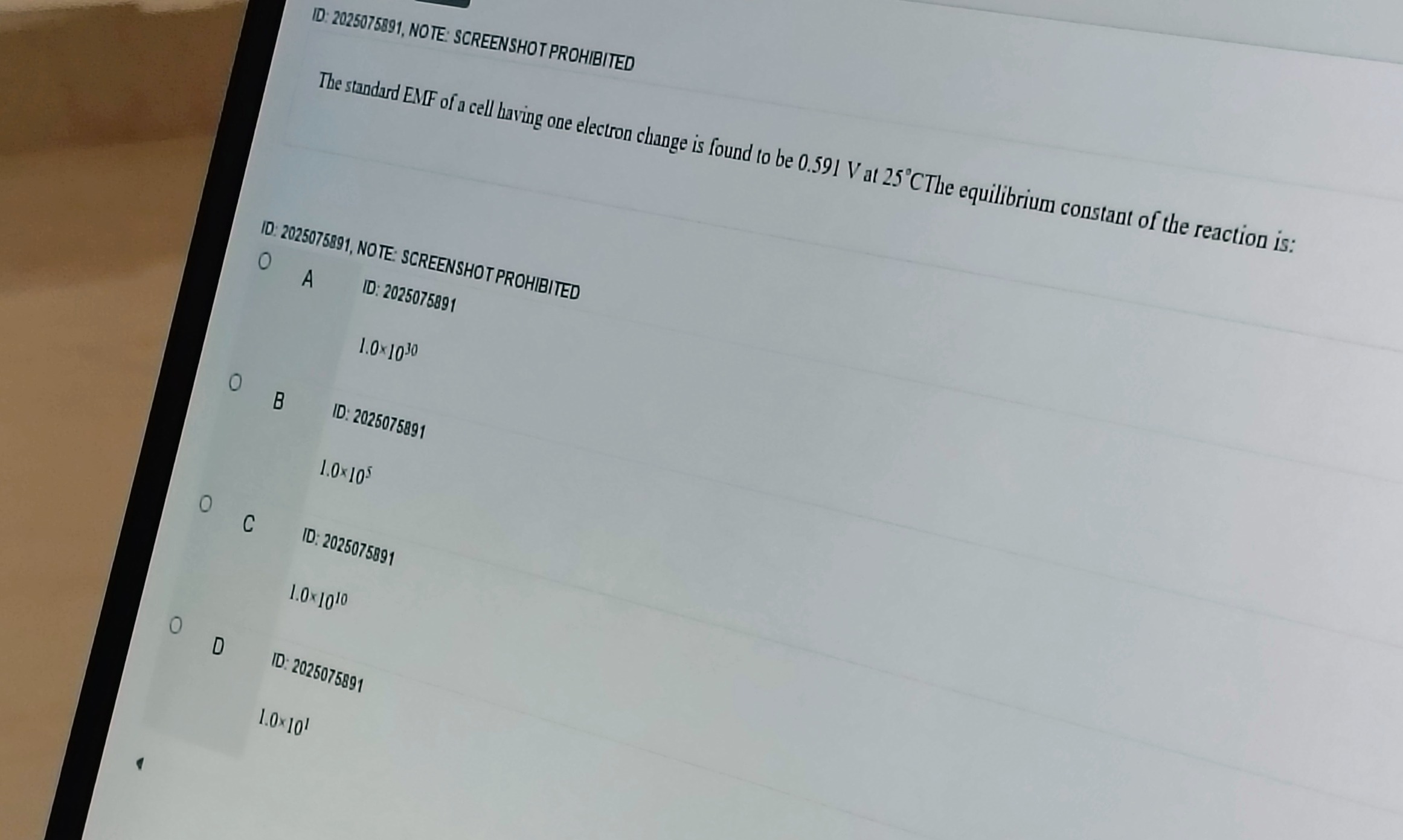
1.0×10³⁰
1.0×10⁵
1.0×10¹⁰
1.0×10¹
1.0 × 10¹⁰
Solution
The relationship between the standard EMF (Ecell∘) and the equilibrium constant (K) of a reaction at 25°C is given by the Nernst equation at equilibrium:
Ecell∘=n0.0591logK
Where:
Ecell∘ = Standard EMF of the cell n = Number of electrons involved in the reaction K = Equilibrium constant
Given:
Standard EMF (Ecell∘) = 0.591 V Number of electrons (n) = 1 Temperature = 25°C
Substitute the given values into the equation:
0.591=10.0591logK
0.591=0.0591logK
To find logK, divide both sides by 0.0591:
logK=0.05910.591
logK=10
To find K, take the antilog of 10:
K=1010
Therefore, the equilibrium constant of the reaction is 1.0×1010.
