Question
Question: If $\eta$ is the coefficient of viscosity and T is temperature in kelvin, a linear plot is obtained ...
If η is the coefficient of viscosity and T is temperature in kelvin, a linear plot is obtained for
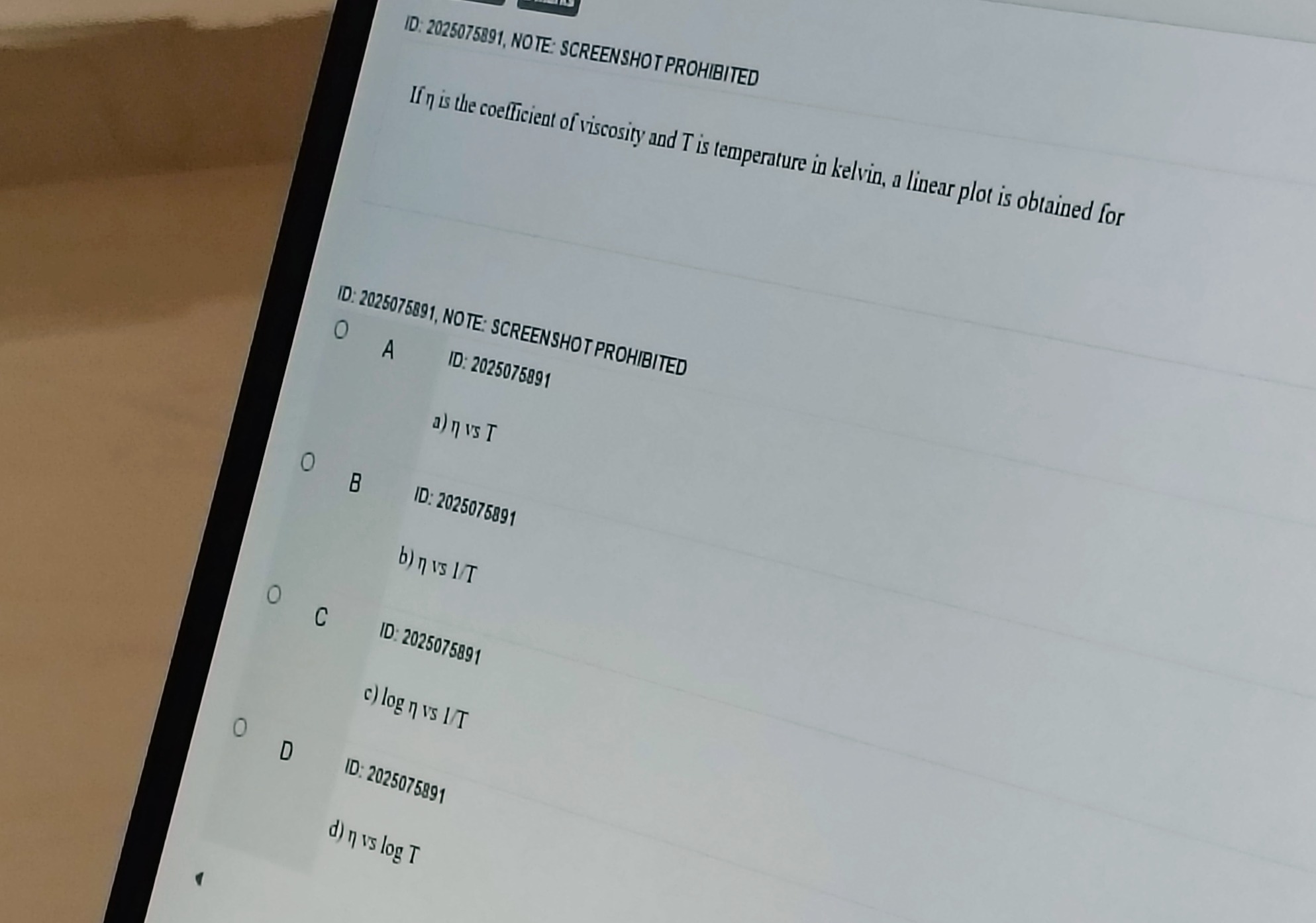
η vs T
η vs 1/T
log η vs 1/T
η vs log T
log η vs 1/T
Solution
The viscosity (η) of a liquid is highly dependent on temperature (T). For most liquids, viscosity decreases exponentially with increasing temperature. This relationship is often described by an Arrhenius-type equation:
η=AeEa/RT
where:
- η is the coefficient of viscosity
- A is a pre-exponential factor (a constant)
- Ea is the activation energy for viscous flow
- R is the ideal gas constant
- T is the absolute temperature in Kelvin
To obtain a linear plot from this exponential relationship, we take the logarithm of both sides of the equation. Taking the natural logarithm (ln):
lnη=ln(AeEa/RT)
lnη=lnA+ln(eEa/RT)
lnη=lnA+RTEa
This equation can be rearranged into the form of a straight line, y=mx+c:
lnη=(REa)(T1)+lnA
Here, if we plot:
- y=lnη (or logη, as log10η=ln10lnη also yields a linear plot)
- x=T1
The plot will be a straight line with:
- Slope (m) =REa
- Y-intercept (c) =lnA
Therefore, a linear plot is obtained when log η is plotted against 1/T.
