Question
Question: A carnot engine, efficiency 30% and temperature of sink 300 K to increase efficiency up to 70% calcu...
A carnot engine, efficiency 30% and temperature of sink 300 K to increase efficiency up to 70% calculate change in temperature of source.
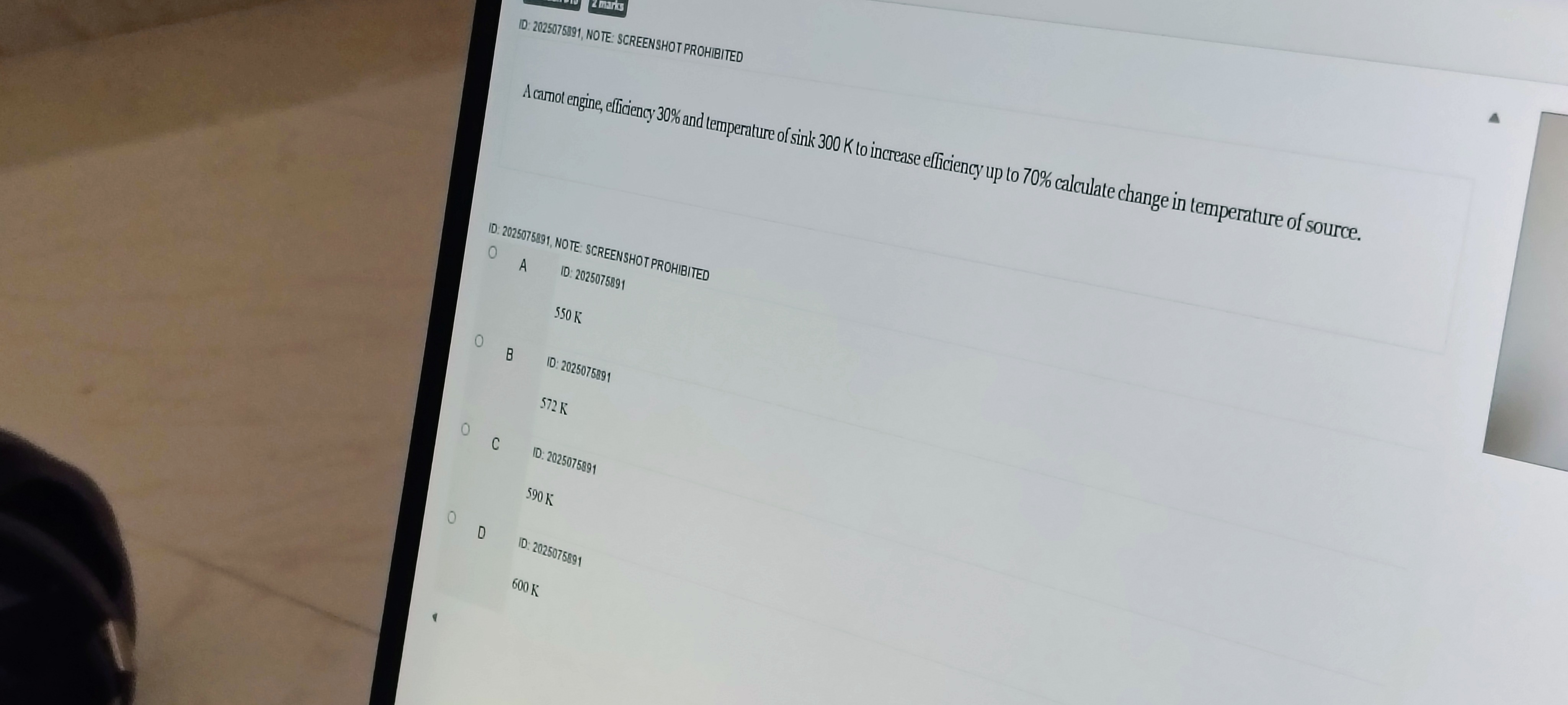
550 K
572 K
590 K
600 K
572 K
Solution
The efficiency of a Carnot engine is given by the formula:
η=1−TsourceTsink
where η is the efficiency, Tsink is the temperature of the cold reservoir (sink), and Tsource is the temperature of the hot reservoir (source). All temperatures must be in Kelvin.
Given: Initial efficiency (η1) = 30% = 0.30 Temperature of sink (Tsink) = 300 K
Step 1: Calculate the initial temperature of the source (Tsource1) Using the efficiency formula for the initial state:
0.30=1−Tsource1300
Tsource1300=1−0.30
Tsource1300=0.70
Tsource1=0.70300=73000K
Step 2: Calculate the final temperature of the source (Tsource2) The efficiency is increased to 70% (η2 = 0.70). The temperature of the sink remains constant at 300 K. Using the efficiency formula for the final state:
0.70=1−Tsource2300
Tsource2300=1−0.70
Tsource2300=0.30
Tsource2=0.30300=33000=1000K
Step 3: Calculate the change in temperature of the source Change in temperature of source (ΔTsource) = Tsource2−Tsource1
ΔTsource=1000−73000
To subtract, find a common denominator:
ΔTsource=71000×7−73000
ΔTsource=77000−3000
ΔTsource=74000K
Now, convert the fraction to a decimal:
ΔTsource≈571.42857K
Comparing this value with the given options, the calculated value 571.42857 K is closest to 572 K.
