Question
Question: (i) Write down any three differences between Lanthanides and Actinides ? (ii) The melting and boi...
(i) Write down any three differences between Lanthanides and Actinides ?
(ii) The melting and boiling points of Zn , Cd and Hg are low. Why ?
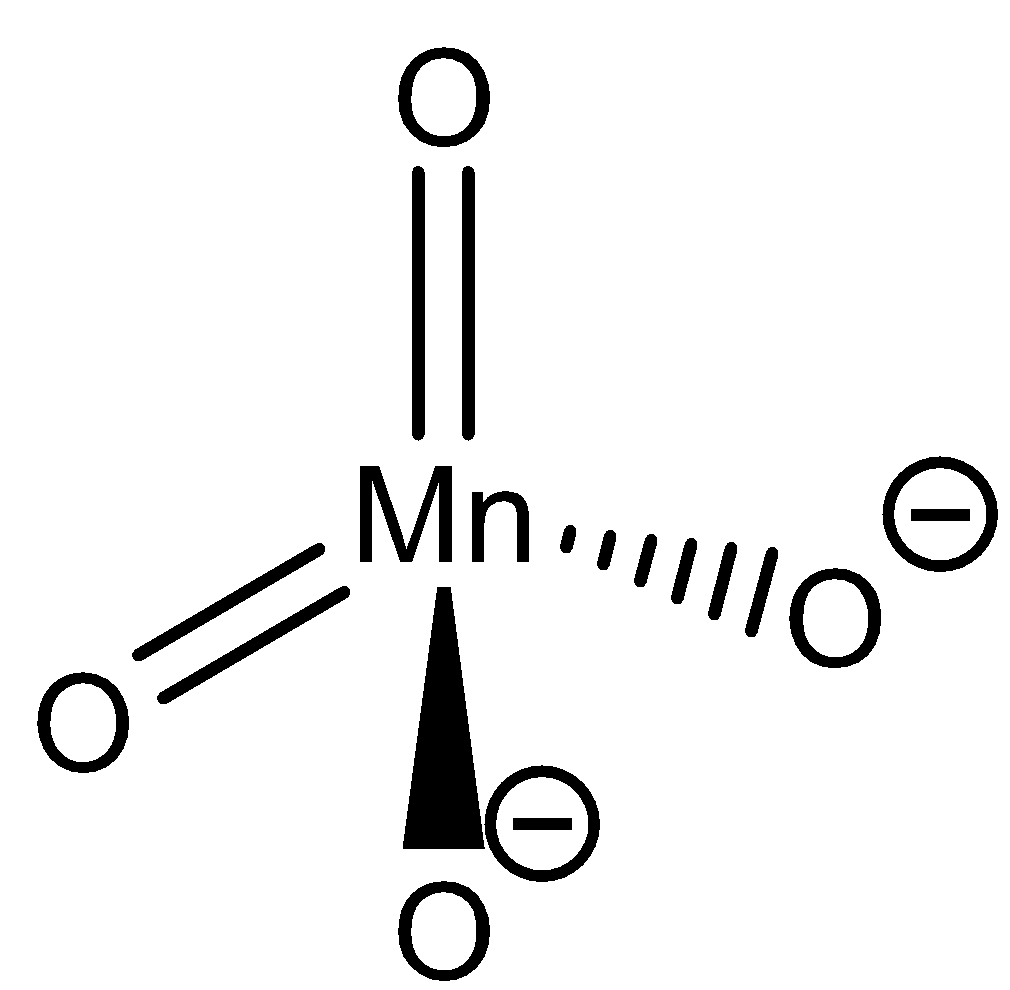
(iii) Draw the structure of manganate ions.
Solution
Hint : Lanthanide and Actinide both constitutes to the f-block elements, and have a different position in the periodic table this is because their periodicity pattern is quite different than the other elements known, and their basic properties mainly differs from the inner-transition elements that is the d-block elements.
Complete step by step solution :
(i) As we know the basics of what these elements are, let's talk about the differences between these two.
The three differences are:
All lanthanides are non-radioactive elements as they occur naturally, whereas the Actinides are radioactive in nature, and have a close relation with nuclear chemistry.
Lanthanides does not form oxo -cations whereas the Actinides have a tendency to form oxo-cations some of the examples are: UO+,PuO+,NpO2+.
Most of the lanthanides are colourless in nature but most of the actinides are coloured compounds or ions.
(ii) Zinc (Zn), Cadmium (Cd) and Mercury ( Hg ) are a part of d-block elements and they exhibits low melting and boiling points as their electronic configuration has a filled d-subshell, and this leads to a weakening of metallic bonds of these elements.
(iii) The structure of Manganate ion is as follows:
The chemical formula of Manganate ion is MnO42−
Note : There are more differences when it comes to Lanthanides and the Actinides, when we talk about the accumulation of electrons in the series, the electrons in Lanthanides are filled in the 4f- orbitals whereas In actinides electrons are filled in the 5f-orbitals, the other difference is the binding energy as the binding energy of 4f electrons is relatively less than that of 5f-electrons.
