Question
Question: i.) VSEPR considerations (any two) ii.) Write the shape of the following molecules \(BeC{{l}_{2}},...
i.) VSEPR considerations (any two)
ii.) Write the shape of the following molecules BeCl2,BF3,CH4,NH3,H2O
iii.) Write any two suggestions of Linus Pauling in valence bond theory
iv.) Explain the formation of NH3 molecule with the help of valence bond theory
v.) Explain the formation of Bells, using valence bond theory - Hybridisation
Solution
VSEPR theory is defined as the electron pairs surrounding the central atom must be arranged in space as far apart as possible to minimize the electrostatic repulsion experienced between them.
Complete step by step answer:
1. Valence shell electron-pair repulsion theory (VSEPR theory) enables us to predict the molecular structure, including approximate bond angles around a central atom, of a molecule from an examination of the amount of bonds and lone electron pairs in its Lewis structure.
2. A lone pair of electrons occupy more space than a bonding pair of electrons because lone pair of electron is under the influence of only one nucleus of the central atom, they are expected to occupy more space with a greater electron density than the bond pair electrons which are under the influence of two nuclei.The decreasing order of repulsion is mentioned below
Lone pair-Lone pair repulsion > Lone pair-Bond pair repulsion > Bond pair- bond pair repulsion
Repulsion forces decrease sharply with increasing interior angle. They are stronger at 90 degree weak at 120 degree and very weak at 180 degree.
- Influence of a bonding electron pair decreases with increasing value of electronegativity of an atom forming a molecule. A central atom can be defined as any atom that is bonded to two or more than two other atoms. The first and the most important rule of the VSEPR theory is that the bond angles about a central atom are those that minimize the total repulsion experienced between the Electron pairs in the atom’s valence shell.
- Multiple bonds behave equivalent to a single electron pair for the purpose of VSEPR bond theory.
The two electron pair of a double bond occupies more space than one electron pair of a single bond.
-The lone pair electrons repel bond pair electrons giving rise to some distortions in the molecular shape.
Hybridization of an atom is identified by calculating the sum of sigma bonds and lone pairs.
- If a sum of sigma bonds and lone pairs is 2 ,sp hybridization
- If a sum of sigma bonds and lone pairs is 3 ,sp2 hybridization
- If a sum of sigma bonds and lone pairs is 4 ,sp3 hybridization
- If a sum of sigma bonds and lone pairs is 5, sp3d hybridization
- If a sum of sigma bonds and lone pairs is 6, sp3d2 hybridization.
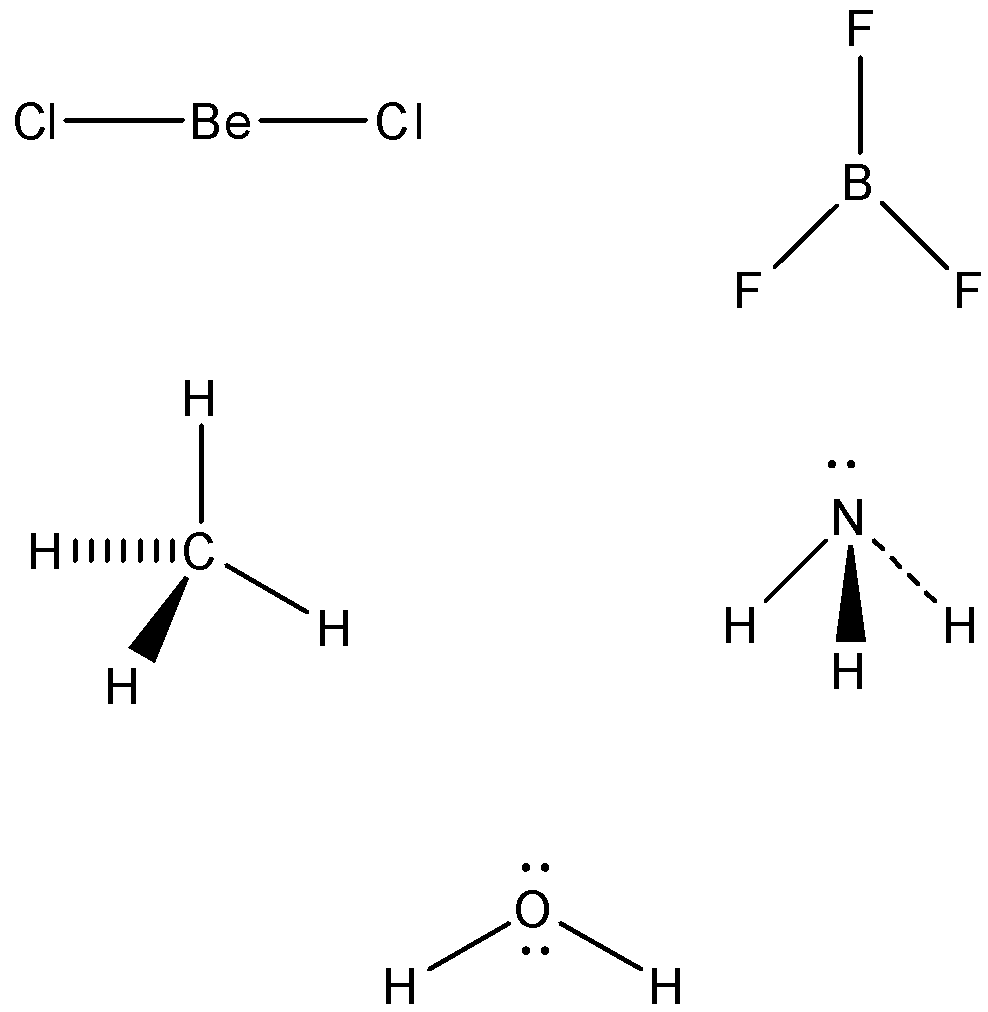
- From above theory the shape of BeCl2 is linear.
- The shape of BF3 is trigonal planar.
- The shape of CH4 is tetrahedral.
- The shape of NH3 is pyramidal.
- The shape of H2O is V- shaped.
Following are the shapes:
3.Pauling's big contribution to chemistry was valence bond theory, which combined his knowledge of quantum mechanical theory along with his knowledge of basic chemical facts, like bond lengths and and bond strengths and shapes of molecules. Valence bond theory, like Lewis's bonding theory, provides a straightforward model that's useful for predicting and understanding the structures of molecules, especially for chemical science.
4. According to valence bond theory, the number of bonds formed by an atom is equal to the number of unpaired electrons present in it.
- Also, only those two atoms make bonds which have a unpaired electron with opposite spins.
- In N2, both the nitrogen atoms have 3 unpaired electrons present in the valence shell, thus they form triple bonds in this molecule.
N≡N.
5. According to the valence bond theory BeCl2 has 2 bond pairs and 0 lone pairs. So it's hybridization is sp. So the shape of BeCl2 is linear.
Note:
As a result of the distortions created different types of shapes are arised :
| Bond pair | Lone pair | Shape |
|---|---|---|
| 2 | 0 | Linear |
| 4 | 0 | Tetrahedral |
| 3 | 1 | Pyramidal |
| 2 | 2 | V shape |
| 5 | 0 | Trigonal bipyramidal |
| 4 | 1 | See saw |
| 3 | 2 | T shape |
| 2 | 3 | Linear. |
