Question
Question: I. Nitrogen \(({N_2})\) reacts with oxygen \(({O_2}),\) the compound \(N{O_2}\) can be formed as a p...
I. Nitrogen (N2) reacts with oxygen (O2), the compound NO2 can be formed as a product.
II. Nitrogen atoms donate four electrons to form N4+ ions and oxygen atoms gain two electrons to form O2− ions when nitrogen and oxygen form an ionic bond.
A. Statement I is true, Statement II is true
B. Statement I is true, Statement II is false
C. Statement I is false, Statement II is true
D. Statement I is false, Statement II is false
Solution
Oxygen forms oxides of nitrogen by reacting with nitrogen. In a molecule, the number of valence electrons are distributed according to acts rule. But nitrogen in this case does not obey that rule. Use this information to solve the above question.
Complete Step by Step Solution:
We distribute a total number of valence electrons in atoms, of molecules, such that each atom should obey acts of rule.
Nitrogen dioxide can be prepared by treating N2 with oxygen as follows
N2+2O2→2NO2
From the above explanation, we can conclude that the statement I is true.
We can draw the structure of NO2 as,
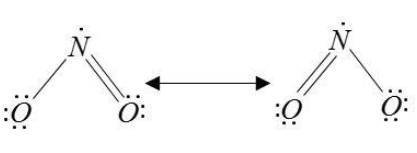
Here we see that the N-atom has an odd electron and does not obey acts rule. Therefore, we cannot be sure that nitrogen atoms will always donate four electrons to form N4+ ions. It will depend on the resonating structure of NO2. Thus, if we consider in general, then statement II is false.
**Therefore, from the above explanation the correct option is (B) Statement I is true, Statement II is false.
Additional Information: **
Nitrogen dioxide gas is brown gas and acidic in nature or yellowish brown liquid when cooled or compact compressed.
Vapors of NO2 gas are heavier than air
NO2 is toxic by inhalation and skin absorption. It is non-combustible but accelerates the burning of combustible materials.
NO2can be prepared by heating pb(NO3)2 to 673k.
2Pb(NO3)2673k4NO2+PbO+O2
Note:
In NO2molecule. Nitrogen is bonded through a single bond with one oxygen and double bond with another oxygen. The bond on the oxygen molecules can change and transfer to each other. So, two structures are formed with the possibility of each oxygen getting a chance to form a double bond with nitrogen. Due to three bonds, the molecule has three electron pairs and is a trigonal planar for electron pair geometry.
