Question
Question: i.Identify the following ii.Give any 2 differences between them. 
Solution
Purification of organic compounds is an essential, although difficult, phase following their extraction from natural sources or laboratory synthesis. Purification of an organic compound is mostly determined by the composition of the compound and the presence of impurities.
Complete answer:
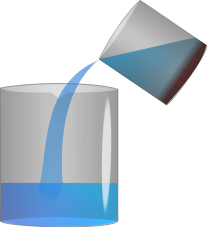 This diagram refers to Decantation.
This diagram refers to Decantation.
Decantation is the isolation of liquid from solids and other immiscible (non-mixing) liquids by separating the liquid layer on top from the solid or liquid layer below. After pouring out the top coat, tip the mixture to complete the process. This procedure can also be used to distinguish two liquids that don't combine, such as oil and water. When we remove the oil and water mixture from the container, two distinct layers form, with water at the bottom and oil, which is lighter, at the top. The oil layer on top can be removed by dumping it into another vessel, leaving the water layer at the bottom.
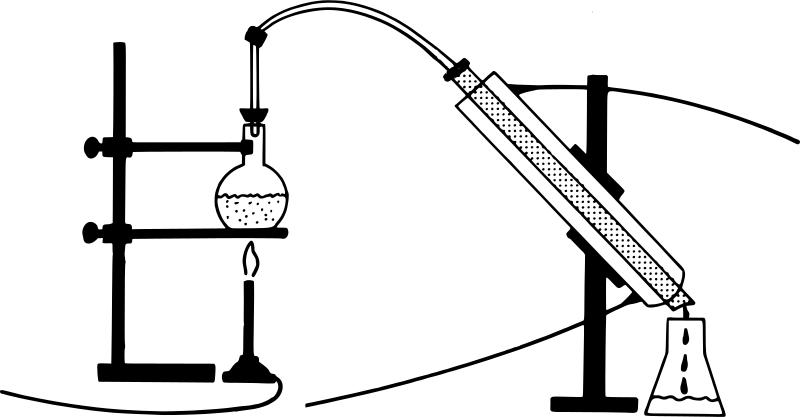
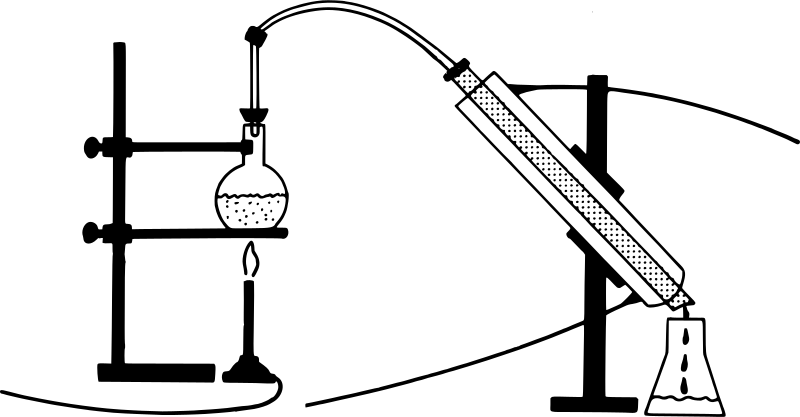 This diagram refers to Distillation.
This diagram refers to Distillation.
Distillation works on the idea that a mixture of liquids may be distinguished by the difference in their boiling points. The temperature at which the liquid's vapour pressure equals ambient pressure is known as the boiling point. This approach distinguishes between volatile and non-volatile liquids. The mixture is then heated in the RB flask. The part that is more liquid or has a lower boiling point evaporates more quickly and is collected in a separate jar. A condenser is a device that speeds up the condensation process.
| DISTILLATION | DECANTATION |
|---|---|
| Decantation is the process of separating a liquid from a solid by pouring the liquid, known as the supernatant, from the mixture while leaving the solid residue undisturbed. | Distillation is a method of separating a homogeneous mixture of liquids by taking advantage of their different boiling points. |
| Differences in boiling points is not required. | Differences in boiling points is required. |
Note:
As a mixture of liquids reaches its boiling point, all of the volatile constituents boil. However, the amount of a constituent in the resulting vapour is determined by its relation to the mixture's overall vapour pressure. As a result, compounds with higher partial pressures can be condensed in vapours, whereas those with lower partial pressures can be concentrated in liquids.
