Question
Question: (i) How is nitric acid manufactured by the Ostwald process? (ii) Write down the reaction of Ozone wi...
(i) How is nitric acid manufactured by the Ostwald process? (ii) Write down the reaction of Ozone with black lead sulphide. (iii) Draw the structure of IF7.
Solution
Nitric acid is manufactured using Ostwald’s process with ammonia as the starting material. Ozone gas is a very good oxidising agent and reacts with lead sulphide resulting in its oxidation while itself getting reduced. In IF7, the central atom undergoes sp3d3 hybridisation.
Complete step by step answer:
(i) Nitric acid is manufactured through Ostwald’s process. It is a two-step process in which ammonia is converted into nitric acid. The first step involves the oxidation of ammonia into nitric oxide and nitrogen dioxide. The nitrogen dioxide formed in the first step is then dissolved in water which leads to the formation of nitric acid.
The first step involves two mini steps:
Step 1: Primary oxidation
Ammonia is oxidised to nitric oxide using oxygen at a temperature of 600oC in the presence of Pt as a catalyst. The reaction is given below:
4NH3(g)+5O2(g)Pt600oC4NO(g)+6H2O(g)
Step 2: Secondary oxidation
The nitric oxide from step 1 is cooled to a temperature of 150oC and is oxidised to nitrogen dioxide at a temperature of 50 oC. The reaction is shown below:
2NO(g)+O2(g)50oC2NO2(g)
The second step involves two mini steps:
Step 1: Absorption of nitrogen dioxide
The nitrogen dioxide is reacted with water in order to give nitric acid. The reaction is given below:
3NO2(g)+H2O(l)→2HNO3(aq)+NO(g)
Step 2: Concentration
To increase the concentration of nitric acid, its vapours are passed over concentrated sulphuric acid; since sulphuric acid is a dehydrating agent, it absorbs the water molecules from nitric acid vapour due to which we get the concentrated nitric acid.
Hence nitric acid is manufactured using Ostwald’s method.
(ii) Lead sulphide reacts with Ozone at room temperature to give lead sulphate and oxygen. The reaction is given below:
PbS(s) Lead(II)sulphate+4O3(g) OzoneRTPbSO4(s) Lead(II)sulphate+4O2(g) Oxygen
(iii) In IF7, the central atom i.e. It undergoes sp3d3 hybridisation and forms seven bonds with seven Fluorine atoms. The geometry of the molecule is pentagonal bipyramidal according to VSEPR theory. Its structure is shown below:
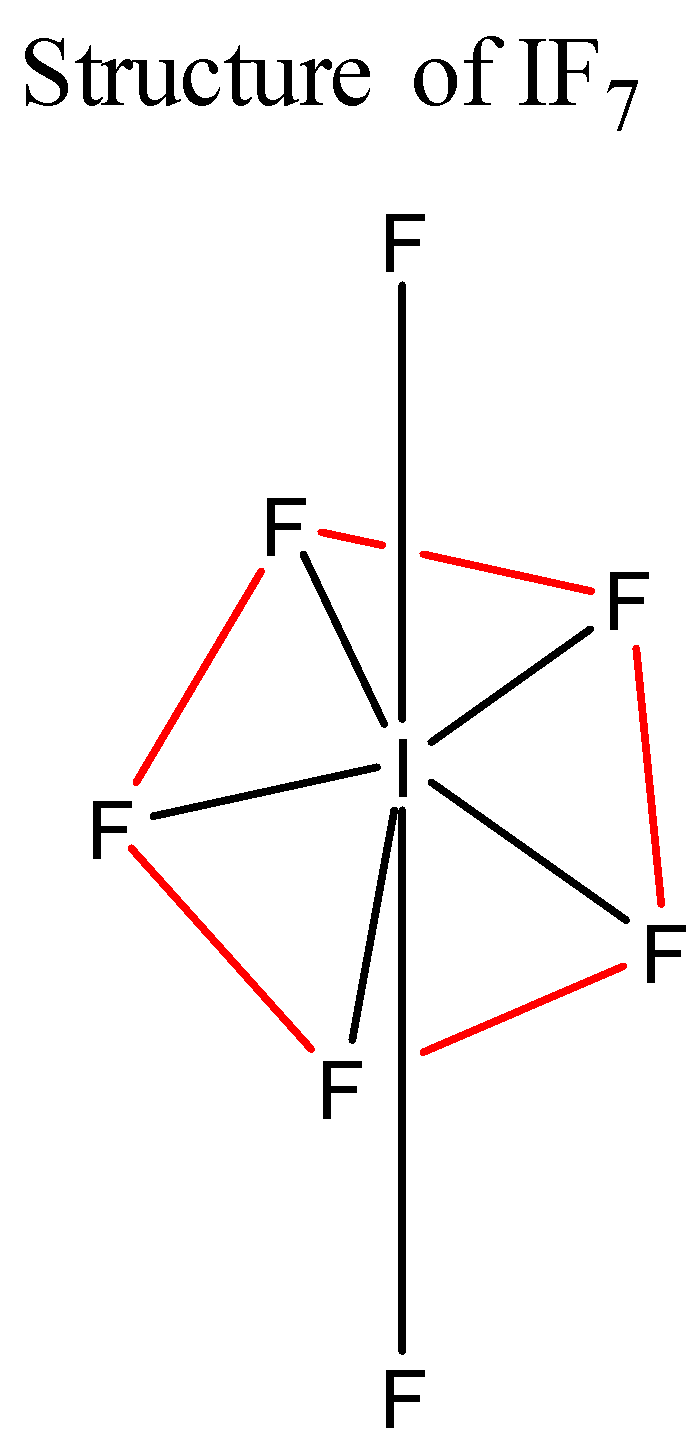
Note: In the molecule IF7 all the bonds are not equivalent. The two axial bonds are shorter than the five equatorial bonds. This is because the s-character in the hybrid orbitals is not the same. The s-character in the axial orbitals is more than in the equatorial orbitals. Greater the s-character in a bond, shorter will be the bond.
