Question
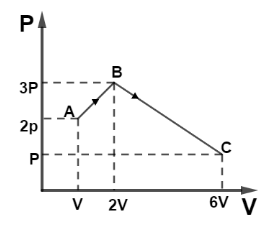
Question: (i) Find total work by the gas during process ABC. (ii) Find \(\Delta {U_{ABC}}\) if the gas is m...
(i) Find total work by the gas during process ABC.
(ii) Find ΔUABC if the gas is monatomic.
Solution
In order to solve this question we need to understand the thermodynamic process. A thermodynamic process is defined as a process in which the state of gas changes, it can be either reversible or irreversible. Reversible process is one in which we can achieve each step involved in state transition while repeating from final state to initial state; these processes are slow and are known as quasi-static processes. Irreversible processes are one step which cannot be achieved while returning back from final to initial state. There are different types of processes like isochoric, adiabatic, isobaric and isothermal processes. Also the first law of thermodynamics is conservation of energy.
Complete step by step answer:
(i) Work done by gas in a changing state is defined as the area under curve P-V. So area under curve ABC can be divided as shown,
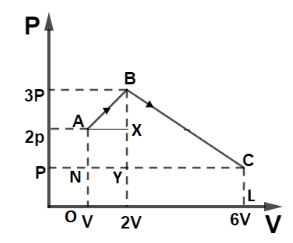
So, Area of ΔAXB is given by, ΔAXB=21(AX)(XB)
From figure, AX=V and XB=P
So, ΔAXB=21PV
Area of ΔBYC is given by, ΔBYC=21(YC)(BY)
From figure, YC=4V and BY=2P
So, ΔBYC=21(4V)(2P)
ΔBYC=4PV
Area of square AXYN is given by, ar(AXNY)=(NY)(AN)
From figure, AN=P and NY=V
So, ar(AXNY)=PV
Area of rectangle ar(NCLO) is given by, ar(NCLO)=(NC)(CL)
From figure, NC=5V and CL=P
So, ar(NCLO)=5PV
So total area is ΔAXB+ΔBYC+ar(AXYN)+ar(NCLO)
Putting values we get, WABC=21PV+4PV+PV+5PV
∴WABC=10.5PV
So work done by the gas is, WABC=10.5PV.
(ii) Internal energy only depends on the final and initial point as it does not depend upon path travelled. Since gas is monatomic, so specific heat at constant volume is given by, CV=23RT
Also Using Ideal gas equation at point A we get, (2P)(V)=nRTA
TA=nR2PV
And using ideal gas equation at point B we get, (P)(6V)=nRTB
TB=nR6PV
So temperature difference is given by, ΔT=TB−TA
Putting values we get, ΔT=nR6PV−nR2PV
ΔT=nR4PV
So internal energy is given by, U=nCVΔT
Putting values we get, U=n(23R)(nR4PV)
∴U=6PV
So internal energy is U=6PV.
Note: It should be remembered that internal energy is path independent but the work done depends on the path travelled. So internal energy only depends upon final and initial points. Also Isothermal processes in which the gas state changes upon keeping temperature constant, isochoric process is one in which gas state changes by keeping volume constant and isobaric process in which gas state changes by keeping pressure constant.
