Question
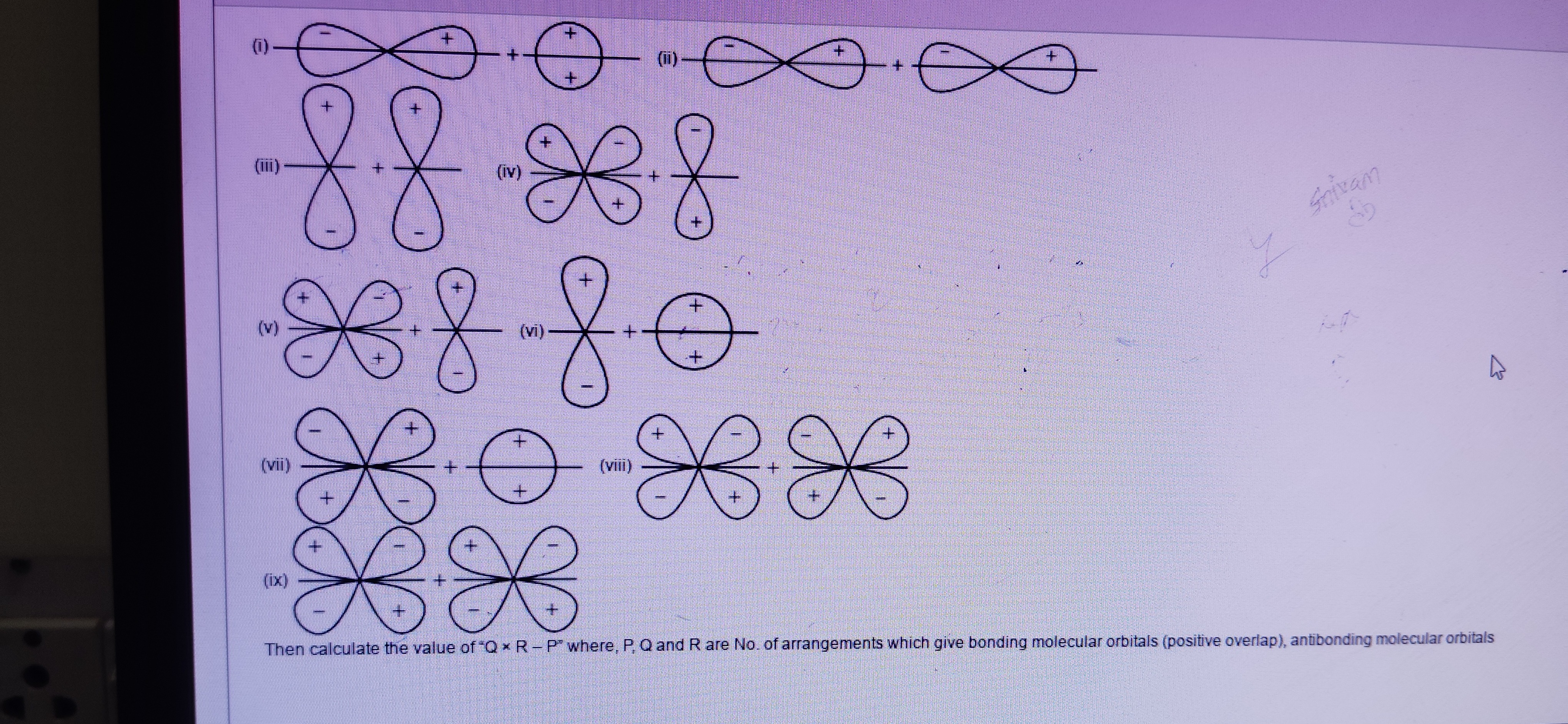
Question: (i) <figure/> (ii) <figure/> (iii) <figure/> (iv) <figure/> (v) <figure/> (vi) <figure/> (vii)...
(i)
(ii)
(iii)
(iv)
(v)
(vi)
(vii)
(viii)
(ix)
Then calculate the value of "Q x R - P" where, P, Q and R are No. of arrangements which give bonding molecular orbitals (positive overlap), antibonding molecular orbitals
2
Solution
The types of overlaps in each diagram are analyzed as follows:
(i) Overlap of a p-orbital (perpendicular to the internuclear axis) and an s-orbital. This is a non-bonding interaction due to cancellation of positive and negative overlaps by symmetry.
(ii) Sideways overlap of two p-orbitals with opposite phases overlapping. This is an antibonding π∗ interaction.
(iii) Head-on overlap of two p-orbitals with the same phase overlapping. This is a bonding σ interaction.
(iv) Overlap of a d-orbital and a p-orbital. Due to symmetry, the net overlap is zero, resulting in a non-bonding interaction.
(v) Overlap of two d-orbitals resulting in a bonding δ interaction.
(vi) Overlap of a p-orbital (along the internuclear axis) and an s-orbital. This is a non-bonding interaction due to cancellation of positive and negative overlaps by symmetry.
(vii) Overlap of a d-orbital and an s-orbital. Due to symmetry, the net overlap is zero, resulting in a non-bonding interaction.
(viii) Sideways overlap of two p-orbitals with the same phase overlapping. This is a bonding π interaction.
(ix) Overlap of two d-orbitals resulting in an antibonding δ∗ interaction.
Based on the analysis:
- Number of bonding molecular orbitals (positive overlap), Q = 3 (from iii, v, viii)
- Number of antibonding molecular orbitals (negative overlap), R = 2 (from ii, ix)
- Number of non-bonding interactions, P = 4 (from i, iv, vi, vii)
We need to calculate the value of Q×R−P.
Q×R−P=3×2−4=6−4=2.
