Question
Question: \((i)\)\[C{O_2}\] is linear where \(S{O_2}\) is bend shaped. \((ii)\) \({H_2}^ + \) ions are more ...
(i)$$$C{O_2}$$ is linear where S{O_2}isbendshaped.(ii){H_2}^ + ionsaremorestablethan{H_2}^ - $ though they have same bond order
Solution
The geometry of the compound depends on the different numbers of electrons in each molecule and the VSEPR (Valence shell electron repulsion) theory. This theory states that the electrons are negatively charged and the valence electrons in different atoms in a molecule repel each other.
Complete answer:
(i) According to the valence shell electron pair repulsion (VSPER) theory, if all the electron pairs around the central atom are not bonded then, the lone pairs distort the shape of the molecule due to the repulsion. In CO2, carbon has 4 electrons in its outermost shell, and oxygen has 6 electrons in its valence shell. The carbon is in the center because of its lower electronegativity, there are no lone pairs present on the atom of carbon, so there will be less repulsion. Therefore, CO2 is a linear structure.
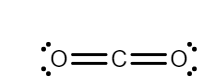
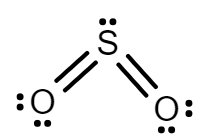
While in SO2, Sulphur has 6 electrons in its valence shell and after forming a bond with oxygen, Sulphur leftover with 1 lone pair. Hence there will be repulsion between bond pairs and lone pairs. So, due to the repulsion structure of SO2 is bent.
(ii) The configuration of H2+ is one electron in 1s bonding orbital, whereas the electronic configuration of H2− is two electrons in 1s bonding orbital and 1 electron in 1s antibonding orbital. So you can say that the bond order for both is 21. But since H2− has more antibonding electrons it will be less stable than H2+ because electrons in antibonding orbitals decrease the stability of the molecule. So H2+ is more stable than H2−.
Note:
Lone pair takes up more space than bonding electrons, as they are only attached to one atom rather than two, so they repel more than bonding electrons. Therefore we can order repulsions between different types of electron pairs: Lone pair-lone pair > bonding pair-lone pair > bonding pair-bonding pair.
The formula for calculating the bond order is:
Bond order= 2(No. of electrons in antibonding Molecular orbit)−(No. of electrons in bonding molecular orbit)
