Question
Question: (I) 1,2-dihydroxybenzene (II) 1,3-dihydroxybenzene (III) 1,4-dihydroxybenzene (IV) Hydroxybenzene. ...
(I) 1,2-dihydroxybenzene (II) 1,3-dihydroxybenzene (III) 1,4-dihydroxybenzene (IV) Hydroxybenzene.
The increasing order of boiling points of above mentioned alcohols is:
A) I < II < III < IV
B) I < II < IV < III
C) IV < I < II < III
D) IV < II < I < III
Solution
The answer here is based on the fact of organic chemistry which is based on the concept of intramolecular hydrogen bonding which is known by drawing the structures and this fact gives you the answer.
Complete answer:
From the lower classes of chemistry, we have studied the types of interaction that exist between the bonds like Van der waals forces, electrostatic force of attraction, intermolecular and intramolecular forces etc.
Now, let us see through the intramolecular forces that are acting in the aromatic alcohols which is basically a hydrogen bonding.
To understand this, let us draw the structures of these molecules.
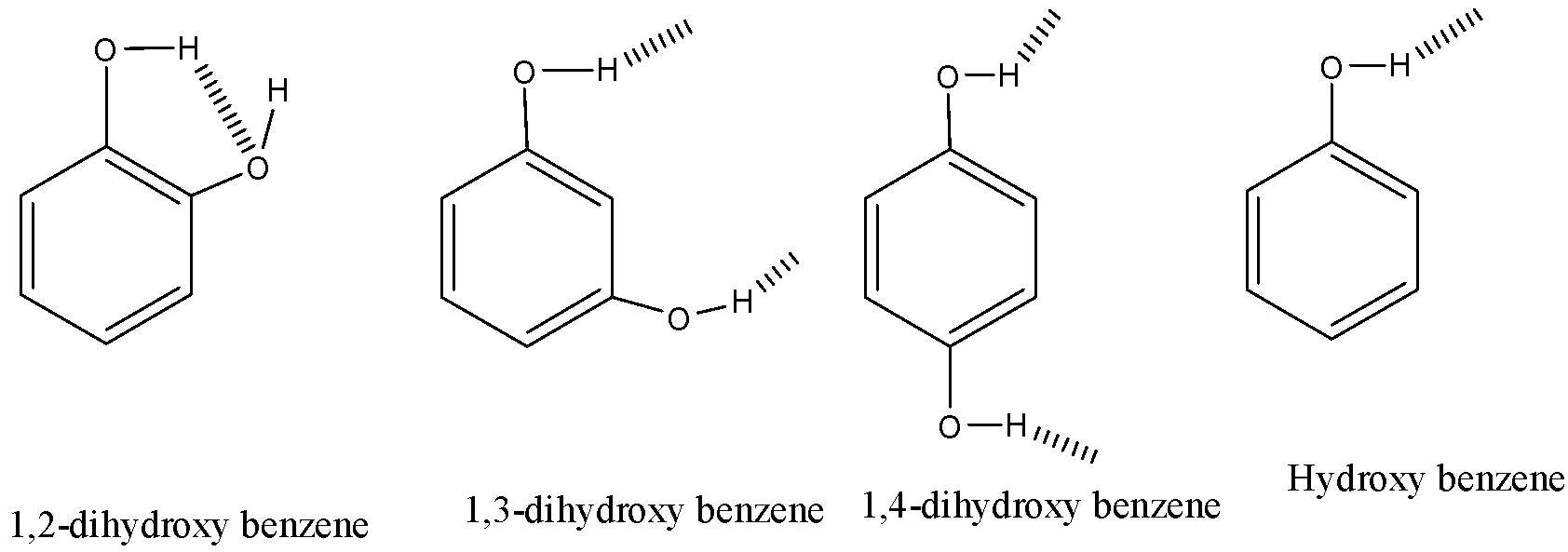
As shown in the above structure,
(i) i,2-dihydroxy benzene shows strong intramolecular hydrogen bonding and the hydrogen easily dissociates here. It shows negligible intermolecular hydrogen bonding
(ii) 1,3-dihydroxy benzene shows strong intermolecular hydrogen bonding and less intramolecular hydrogen bonding as the two alcohol groups are far away.
(iii) 1,4-dihydroxy benzene stronger intermolecular hydrogen bonding compared to 1,3-dihydroxybenzene and negligible intramolecular hydrogen bonding.
(i) Hydroxy benzene has only one alcohol group and shows low intermolecular hydrogen bonding than the 1,3 and 1,4-dihydroxy benzene.
Thus, the boiling point is more for the one which has the highest intermolecular hydrogen bonding because it has strong bonds which are not easily dissociated.
Therefore the boiling points are in the order, 1,4-dihydroxybenzene >1,3-dihydroxybenzene > 1,2-dihydroxy benzene > Hydroxy benzene
Thus, the correct answer is option C) IV < I < II < II < III.
Note: Note that intramolecular and intermolecular have a difference and do not be confused with this definition. Intramolecular means within the same molecule and intermolecular means between two same or different molecules.
