Question
Question: (I)- 1,2-dihydroxybenzene (II)- 1,3-dihydroxybenzene (III)- 1,4-dihydroxybenzene (IV)- Hydroxy...
(I)- 1,2-dihydroxybenzene
(II)- 1,3-dihydroxybenzene
(III)- 1,4-dihydroxybenzene
(IV)- Hydroxybenzene
The increasing order of boiling points of above mentioned alcohol is:
(a)- I < II < III < IV
(b)- I < II < IV < III
(c)- IV < I < II < III
(d)- IV < II < I < III
Solution
Hint In hydroxy benzenes, the boiling point depends on the intermolecular hydrogen bonding and intramolecular hydrogen bonding factors. The compounds having intermolecular hydrogen bonding will have more boiling points and the compounds having intramolecular hydrogen bonding will have a lesser boiling point.
Complete step by step answer:
Dihydroxybenzene means there are two alcohol groups on the benzene ring, if the compound is 1,2-dihydroxybenzene, then the alcohol groups are present on the ortho position. If the compound is 1,3-dihydroxybenzene, then the alcohol groups are present in the meta position. If the compound is 1,4-dihydroxybenzene, then the alcohol groups are present in the para position.
The structure of 1,4-dihydroxybenzene is given below:
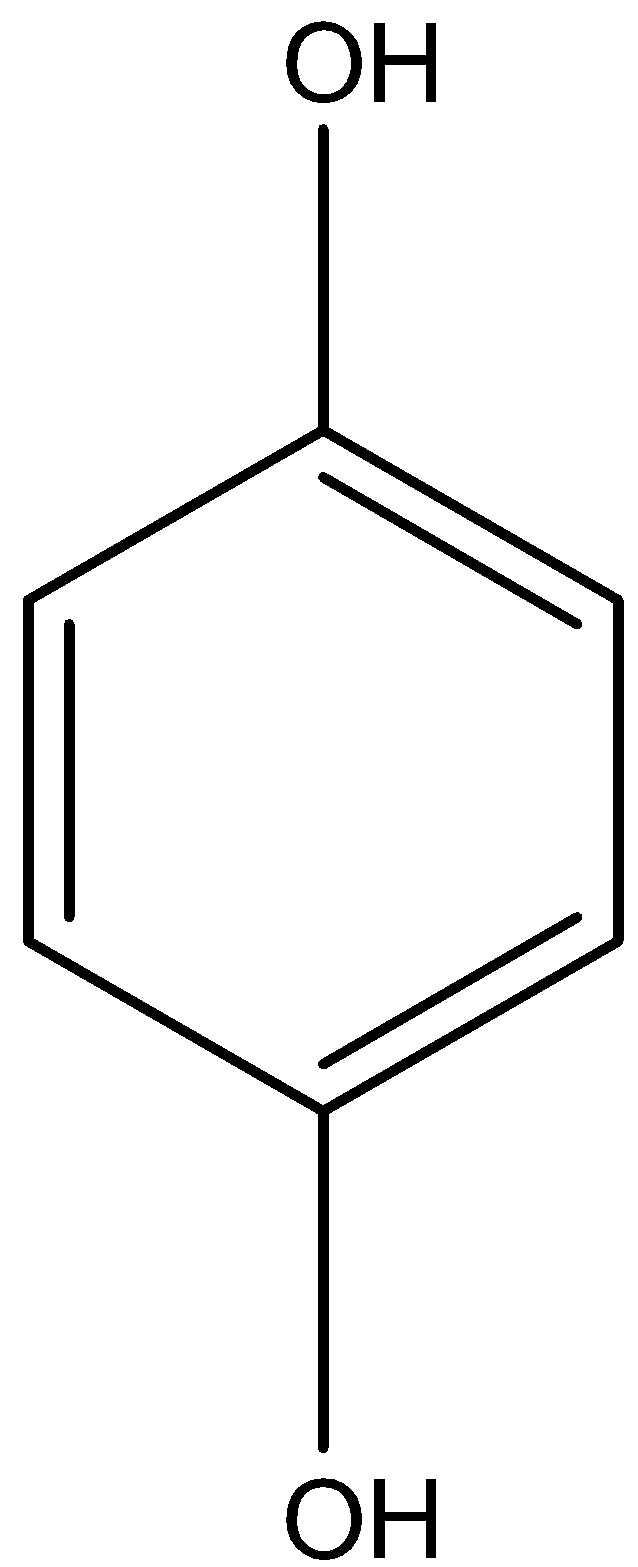
In this compound the alcohol groups can have only intermolecular hydrogen bonding and don't have intramolecular hydrogen bonding because both the alcohol groups are very far from each other. So, it will have the highest boiling point.
The structure of 1,2-dihydroxybenzene is given below:
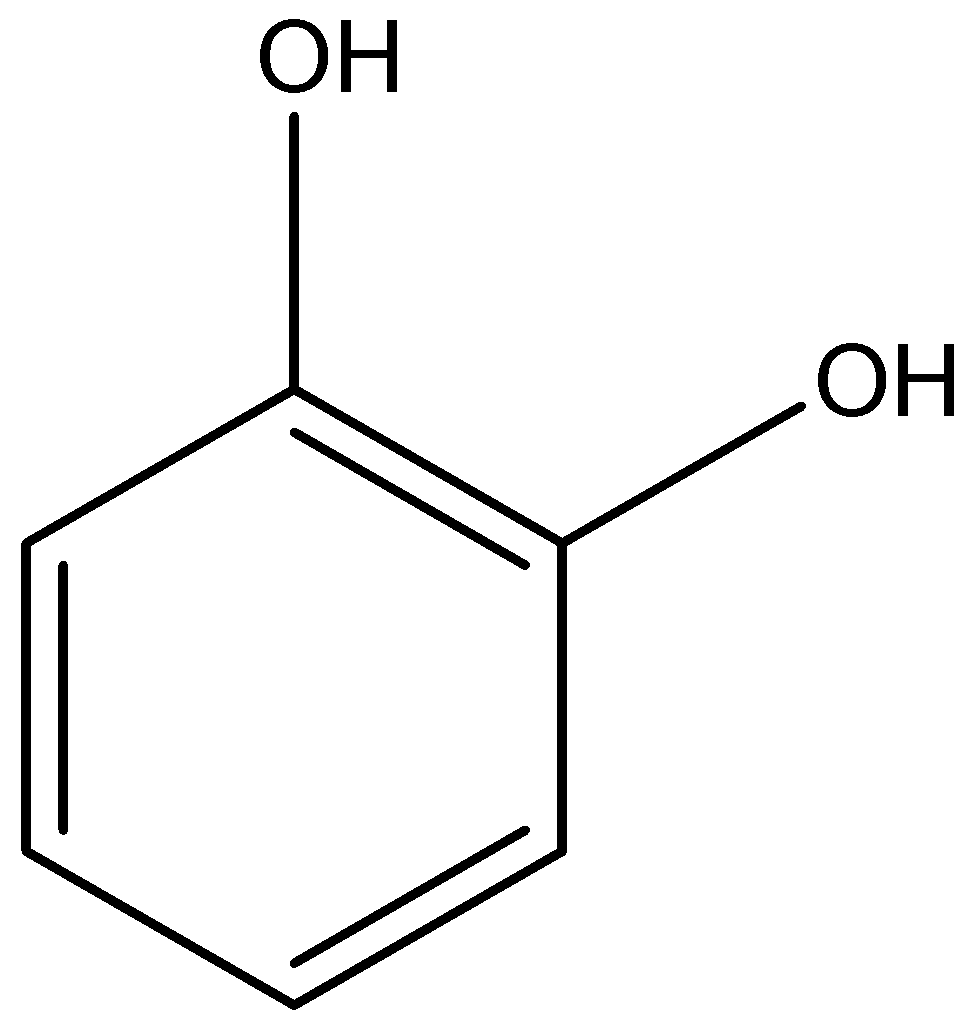
The 1,2-dihydroxybenzene will have an intramolecular hydrogen bonding atom because both the alcohol groups are very close to each other. So, the boiling point of 1,2-dihydroxybenzene will be less than 1,4-dihydroxybenzene.
The structure of 1,3-dihydroxybenzene is given below:
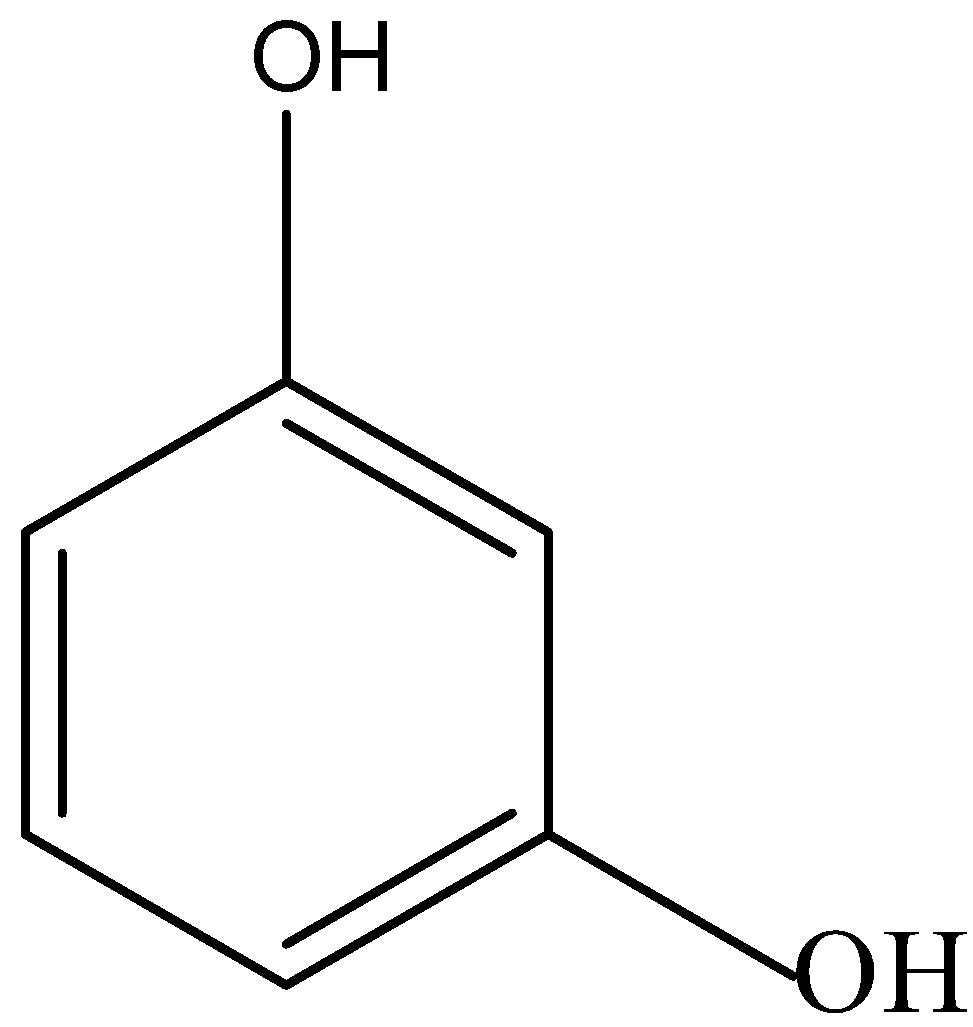
In 1,3-dihydroxybenzene, there is partial intramolecular hydrogen bonding as well as intermolecular hydrogen bonding, so its boiling point is lower than 1,4-dihydroxybenzene and higher than 1,2-dihydroxybenzene.
The structure of Hydroxybenzene is given below:
The Hydroxybenzene will not have any type of hydrogen bonding, so it will have the lowest boiling point.
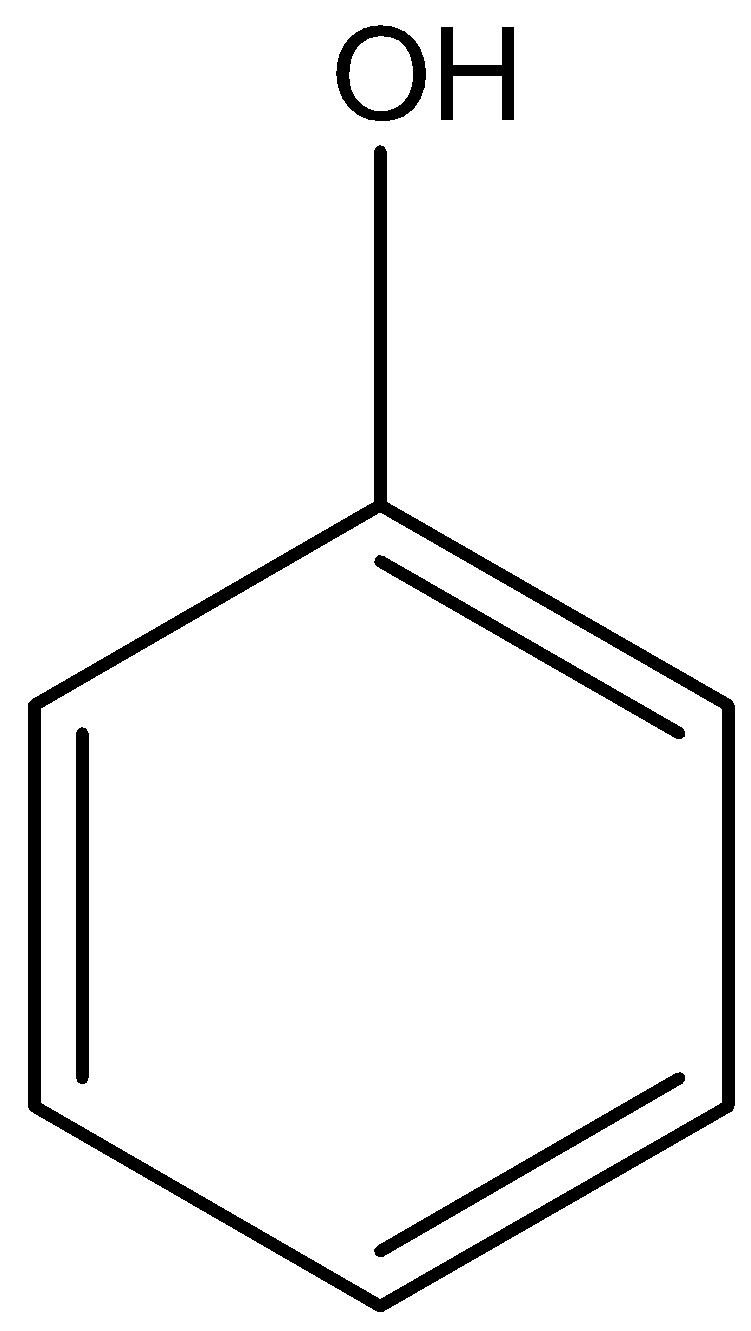
The order will be IV < I < II < III. Therefore, the correct answer is an option (c).
Note: The intermolecular hydrogen bonding means the bonding is formed between different molecules and it is one of the strongest bonds, and the intramolecular hydrogen bonding means the bonding is formed within the molecule.
