Question
Question: Hydrolysis of 1 mol of peroxodisulphuric acid produces: A. 2 mol of sulphuric acid. B. 2 mol of ...
Hydrolysis of 1 mol of peroxodisulphuric acid produces:
A. 2 mol of sulphuric acid.
B. 2 mol of peroxomo sulphuric acid
C. 1 mol of sulphuric acid and 1 mole of peroxomo sulphuric acid
D. 1 mol of sulphuric acid, 1 mole of peroxomo sulphuric acid and 1 mol of hydrogen peroxide
Solution
THydrolysis is a reaction where addition of water takes place. On hydrolysis of one mol of Peroxodisulphuric acid, water molecules break the peroxodisulphuric acid into two new products.
Complete step by step answer:
Hydrolysis is a chemical reaction where water is used as one of the reactants to break the chemical bond present between the atoms of the other reactant molecule.
Peroxodisulphuric acid is an inorganic compound which has a chemical formula of H2S2O8. It is also named as Marshall’s acid which was named after its inventor Professor Hugh Marshall. It is a Sulphur oxoacid which is used as an oxidizing agent. In Peroxodisulphuric acid, the Sulphur molecule is present in +6 oxidation state and a peroxide group.
On hydrolysis of 1 mol of Peroxodisulphuric acid, water molecules break the peroxodisulphuric acid into two new products.
The reaction for the hydrolysis of 1 mol of Peroxodisulphuric acid is shown below.
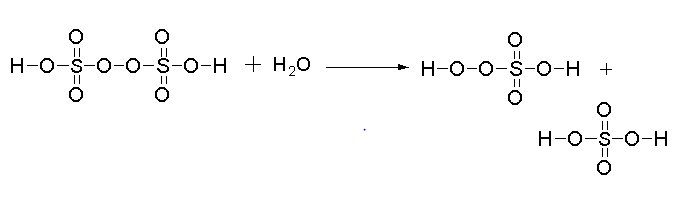
In this reaction one mole of peroxodisulphuric acid reacts with one mole of water to give one mole of peroxomo sulphuric acid and one mole of sulphuric acid.
Therefore, the correct option is C.
Note: The peroxodisulphuric acid is formed by reacting chlorosulfuric acid with hydrogen peroxide where hydrochloric acid is formed as the side product.
