Question
Question: Hydrogen peroxide is prepared in the laboratory by: A.Adding \(Mn{O_2}\) to dilute \({H_2}S{O_4}\)...
Hydrogen peroxide is prepared in the laboratory by:
A.Adding MnO2 to dilute H2SO4
B.Passing CO2 into a paste in cold water
C.Adding PbO2 to an acidified KMnO4
D.Adding Na2O2to cold water
Solution
To answer this question, you should recall the concept of preparation of hydrogen peroxide. It contains oxygen in an intermediate oxidation state and can thus, act as both oxidising and reducing reagent.
Complete step by step answer:
We know that peroxide is a compound which has two oxygen atoms bonded together. And Hydrogen Peroxide is the simplest peroxide. The chemical formula for hydrogen peroxide is H2O2. The structure of the molecule can be drawn as:
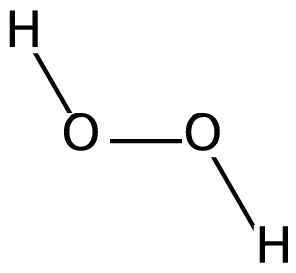
Now we know the methods of preparation of H2O2 so let us analyse each of the options systematically:
A.A balanced chemical reaction for this process can be written as 2MnO2+ 2H2SO4→2MnSO4+ O2+ 2H2O.
We can see that H2O2is not being formed in this reaction hence the given option is wrong and can be eliminated.
B.CO2+H2O→H2CO3.
We can see that H2O2is not being formed in this reaction, thus the given option is wrong and can be eliminated.
C.5PbO2+2Mn2⊕+4H⊕→5Pb2⊕+2MnO4−+2H2O.
It is evident that H2O2 is not being formed in this reaction, the given option is wrong and can be eliminated
D.Na2O2(s)+H2SO4→Na2SO4(s)+H2O2(l).
We can see that here H2O2is being produced. This is indeed a preparation method of laboratory preparation of hydrogen peroxide by the action of ice-cold, dilute sulphuric acid on sodium peroxide or hydrated barium peroxide. A calculated quantity of sodium peroxide is added in small quantities to 20%ice-cold solution of sulphuric acid. Most of the sodium sulphate separates on cooling as crystals of Na2SO4.10H2O. From this reaction, we obtain a 30% hydrogen peroxide yield containing a small amount of sodium sulphate.
Hence, we can conclude that the correct answer to this question is option D
Note: You must remember all the preparation methods of hydrogen peroxide which are most commonly used in the laboratories. Apart from the above reaction, the other important reaction for laboratory preparation of hydrogen peroxide is from barium peroxide: BaO2.8H2O+ H2SO4→ BaSO4+ H2O2+ 8H2O
