Question
Question: Hydrazobenzene on treatment with \({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\) forms:...
Hydrazobenzene on treatment with H2SO4 forms:
A) Azobenzene
B) Azobenzene sulfonic acid
C) Benzidine
D) None of the above
Solution
To solve this we must know that hydrazobenzene is also known as diphenylhydrazine. The structure is two benzene rings to which −NH groups are attached. These two rings are then connected through the nitrogen-nitrogen (N−N) bond. H2SO4 which is known as sulphuric acid creates an acidic medium. A rearrangement reaction occurs.
Complete solution:
We know that hydrazobenzene as the name suggests hydrazo group is −NH group and benzene indicates that this hydrazo group is attached to the benzene ring.
Hydrazobenzene is two benzene rings to which −NH groups are attached. These two rings are then connected through the nitrogen-nitrogen (N−N) bond.
The structure for hydrazobenzene is as follows:
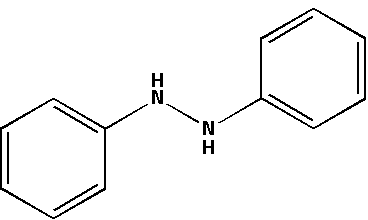
H2SO4 which is known as sulphuric acid creates an acidic medium. Hydrazobenzene in an acidic medium accepts protons from the sulphuric acid. In an acidic medium, hydrazobenzene undergoes a rearrangement reaction.
The mechanism of the reaction when hydrazobenzene reacts with sulphuric acid i.e. H2SO4 is as follows:
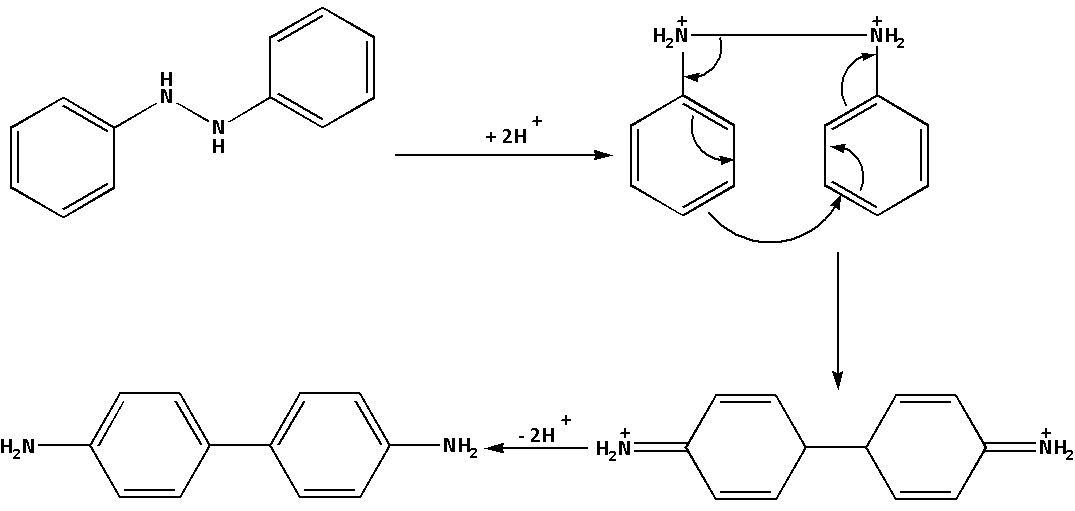
In the reaction, where hydrazobenzene reacts with sulphuric acid i.e. H2SO4 a rearrangement reaction leads to the formation of benzidine. Thus, benzidine is a product of rearrangement of hydrazobenzene in an acidic medium.
Benzidine is not a naturally occurring substance and is synthesized mainly from hydrazobenzene on reacting it with sulphuric acid i.e. H2SO4.
Thus, hydrazobenzene on treatment with H2SO4 forms benzidine.
Thus, the correct option is (C) benzidine.
Note: Benzidine is greyish-red, yellowish or white coloured powder. Benzidine can also be synthesized from nitrobenzene. Nitrobenzene is first converted to diphenylhydrazine or hydrazobenzene using iron powder as a reducing agent which then undergoes the same rearrangement reaction in acidic medium as shown above.
