Question
Question: Hybridization in \({\text{S}}{{\text{O}}_2}\) molecule is: A. \({\text{sp}}\) B. \({\text{s}}{{\...
Hybridization in SO2 molecule is:
A. sp
B. sp2
C. sp3
D. sp3d
Solution
Covalent compounds are formed when the electrons are shared to complete the octet. Sulfur and oxygen are non-metals. They have very high electronegativity. Thus they form a covalent bond. While an ionic bond is formed between metal and non-metal.
Complete step by step answer: There are four types of chemical bonding-ionic, covalent, hydrogen and metallic bonding. Covalent bonding is generally formed between two nonmetals. It is a type of chemical bond in which there is mutual sharing of electrons between two atoms. It is further classified into single, double and triple covalent bonds with respect to mutual sharing of one, two and three bonds respectively.
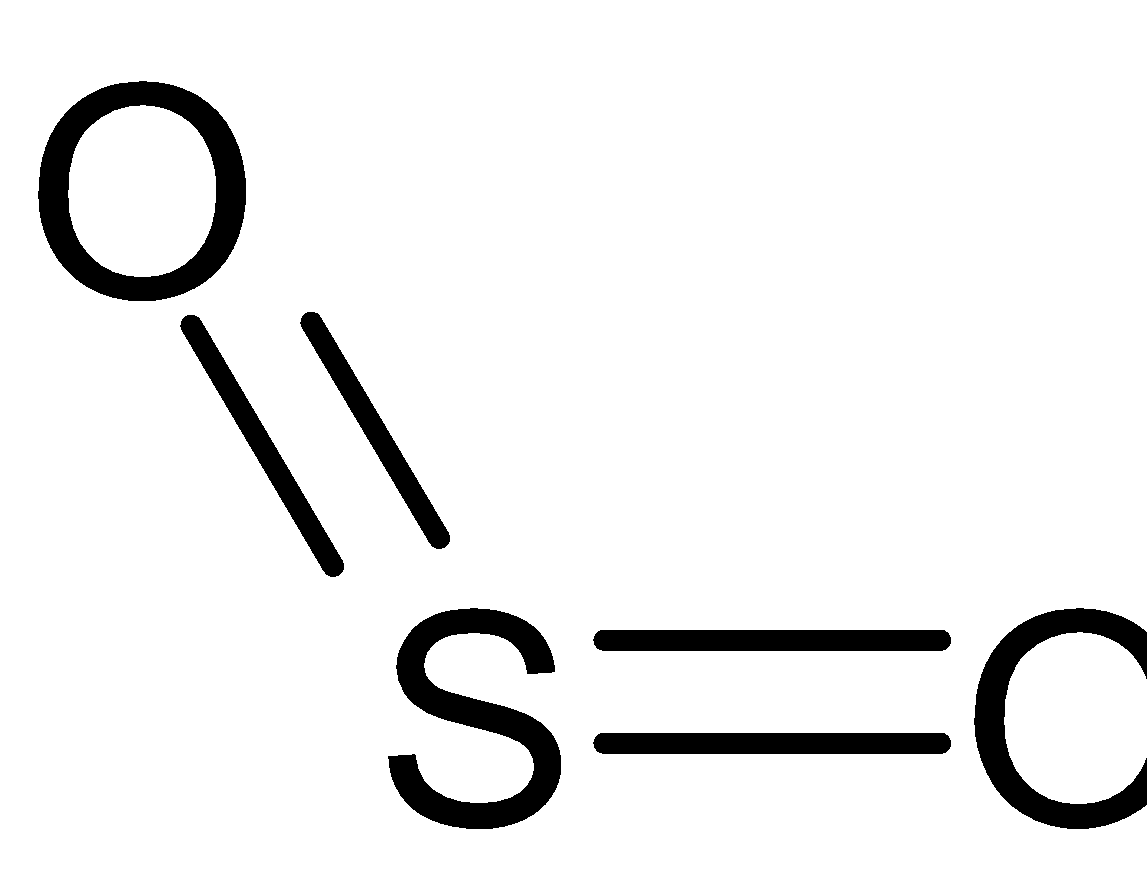
Consider SO2 molecule in which sulfur has six electrons in a valence shell. So to form an octet, it is easy to gain two electrons than lose six electrons. Therefore its valency is two. Similarly, the valency of oxygen is two. The electronic configuration of sulfur is 1s22s22p63s23p4. First two shells are completely filled. There are two electrons in 3s orbital four electrons in 3p orbital and it needs four unpaired electrons. One electron in 3px jumps to 3d orbital. This leads to formation of one unpaired electron in 3d orbital and three unpaired electrons in 3p orbital. Two 3p orbitals and one 3s orbital gets hybridized, thereby forming three sp2 hybrid orbitals. Two hybrid orbitals form σ bonds with oxygen. While 3d and 3p orbitals are involved in the formation of π bonds. Moreover, the hybridization of oxygen is also sp2 . Therefore it forms sp2 hybridization.
Hence the correct option is B.
Note: The molecular geometry of SO2 is V-shaped or bent and the bond angle is 119∘. Two double bonds are there and a lone pair of electrons is there in one hybrid orbital, thereby forming bent shape. The structure of SO2 molecule is given below: