Question
Question: Hybridization and geometry of \({{\text{[Ni(CN}}{{\text{)}}_{\text{4}}}{\text{]}}^{{\text{2 - }}}}\)...
Hybridization and geometry of [Ni(CN)4]2 - are:
a.) sp2d and tetrahedral
b.) sd3and square planar
c.) sp3 and tetrahedral
d.) dsp2 and square planar
Solution
Hint: If we know the concept of hybridization, then we can easily find out the geometry of the compound. After drawing the excited electronic configuration, we can find out the hybridization easily. Also, there is a particular geometry associated with a type of hybridization.
Complete step by step answer:
First of all we have to find out the oxidation state of central metal atom Ni. That is, x+(4×(−1))=−2⇒x=+2. Therefore the oxidation state of Nickel is +2. So, let us draw subshell electronic configuration of Ni + 2:
Ni + 2: 1s22s22p63s23p63d8 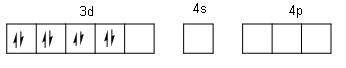
Here, one 3d orbital, one 4s orbital and three 4p orbitals are free. Out of which we need four orbitals because we have four CN - ligands. So, the four orbitals are one 3d, one 4s and two 4p. Therefore, the hybridization is dsp2. For the formation of square planar geometry, two unpaired d-electrons are paired up due to energy made by the approach of ligands, making one of the 3d orbitals empty.
So, the hybridization is dsp2 and the geometry is square planar. Therefore, the correct option is (d).
Additional Information:
We know that hybridization is the concept of mixing two atomic orbitals with the same energy levels to give a degenerated new type of orbitals. This intermixing is based on quantum mechanics.
The atomic orbitals having the same energy level can only take part in hybridization and both full filled and half filled orbitals can also take part in this process.
Note: Whenever you are drawing the electronic configuration of the central metal atom, you need to pair up the unpaired electrons in the presence of strong ligands, otherwise you will not get the right hybridization.
