Question
Question: Hybridization and geometry of \[{\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }}\] are: A.\(s...
Hybridization and geometry of [Ni(CN)4]2− are:
A.sp2d and tetrahedral
B. sd3 and square planar
C. sp3 and tetrahedral
D. dsp2 and square planar
Solution
To solve this question, we must first understand the basic concepts about Hybridization in Coordination Compounds. Then we need to assess the geometrical properties of the Coordinate complexes then only we can conclude the correct answer.
Complete step-by-step answer: Before we move forward with the solution of this given question, let us first understand some basic concepts:
Hybridization: Redistribution of the energy of orbitals of individual atoms to give orbitals of equivalent energy happens when two atomic orbitals combine to form hybrid orbital in a molecule. This process is called hybridization. The new orbitals thus formed are known as hybrid orbitals.
Step 1: Consider the molecule [Ni(CN)4]2− : It has a square planar geometry formed by dsp2 hybridization and not tetrahedral by sp3 . [Ni(CN)4]2− is diamagnetic, so Ni2+ ion has 3d8 outer configuration with two unpaired electrons. For the formation by sp3 hybridization, the 3d orbital would remain unaffected, consequently, the complex would be paramagnetic like Ni2+ ion itself.
Step 2: And we know that, for the formation of square planar structure by dsp2 hybridization, two unpaired d-electrons are paired up due to energy made available by the approach of ligands, making one of the 3d orbitals empty. By this, there is no unpaired electron and the complex would be diamagnetic.
Step 3: The hybridization is as follows:
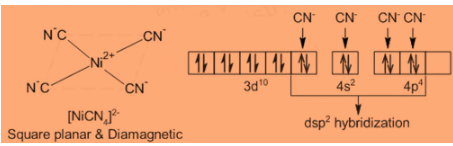
So, clearly we can conclude that the correct answer is Option D.
Note: During the process of hybridization, the atomic orbitals of similar energy are mixed together such as the mixing of two ‘s’ orbitals or two ‘p’ orbital’s or mixing of an ‘s’ orbital with a ‘p’ orbital or ‘s’ orbital with a ‘d’ orbital.
