Question
Question: Hybridisation and shape of \( Xe{F_4} \) is: (A) \( s{p^3}d \), Trigonal bipyramidal (B) \( s{p^...
Hybridisation and shape of XeF4 is:
(A) sp3d, Trigonal bipyramidal
(B) sp3, Tetrahedral
(C) sp3d2, Square planar
(D) sp3d2, Hexagonal
Solution
To determine the shape and the geometry of the molecule, we use VSEPR theory. According to VSEPR theory, the force of repulsion between the lone pairs of electrons on the central atom should be minimal. This is to stabilize the molecule.
Complete answer:
Let’s solve this question using VSEPR theory and the valence shell of the xenon, Xe .
Since, we know that our central atom is xenon, Xe and it has 8 valence electron in the valence shell, and out of these 8 electrons, 6 electrons are in the 5p orbital and the 2 electrons are in the 5s orbital.
Also, the 5d and the 5f orbitals of the xenon are empty. So during the formation of XeF4 , the two excited electrons of the 5p orbital move to the empty 5d orbital. As a result, there are 4 unpaired electrons, 2 in the 5p orbital and 2 in the 5d orbital.
This all over arrangement of electrons in the s,p,d orbitals gives sp3d2 hybridisation of the molecule.
Let’s look at the structure too carefully for better understanding.
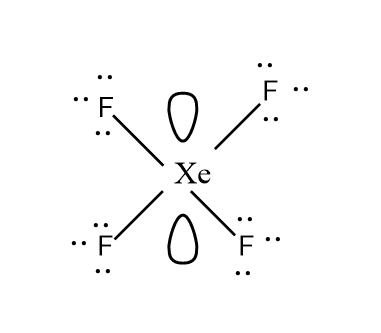
And according to the VSEPR theory, the molecule with sp3d2 hybridisation has square planar geometry.
So, based on the above discussion, the correct option is (C). Therefore, Hybridisation and shape of XeF4 is sp3d2 and square planar respectively.
Note:
The four fluorine atoms pairs with the four half-filled orbitals (i.e. 5p and the 5d orbital) and they lie at the corners of the central atom to minimise the repulsion. Also, the two lone pairs of electrons of the central atom i.e. xenon lie perpendicular to the plane to make the molecule overall stable. Hence, this gives the molecule its square planar shape.
