Question
Question: 
Reactant in the given reaction is:
(A) Isopropyl alcohol
(B) Butanaldehyde
(C) Acetone
(D) None of these
Solution
The carbon which can accept the lone pair of electrons of hydrazine due to its electrophilic nature that carbon will react readily with hydrazine and gives alkane as a product followed by abstraction of base from ethanoate.
Complete step by step answer:
As we know that the carbonyl carbon is electrophilic in nature due to oxygen attached to it, which attracts the electrons of carbon towards itself. So now hydrazine is an electron rich compound which attacks on electrophilic carbon and forms hydrazone by eliminating water. Now the base which is sodium ethanoate in this reaction abstracts a proton from hydrazone and generates carbanion at carbonyl carbon. This carbanion itself abstracts protons from water. This process is repeated and hydrogen is attached to carbonyl carbon. So now the product we get is propane as shown in the mechanism below.
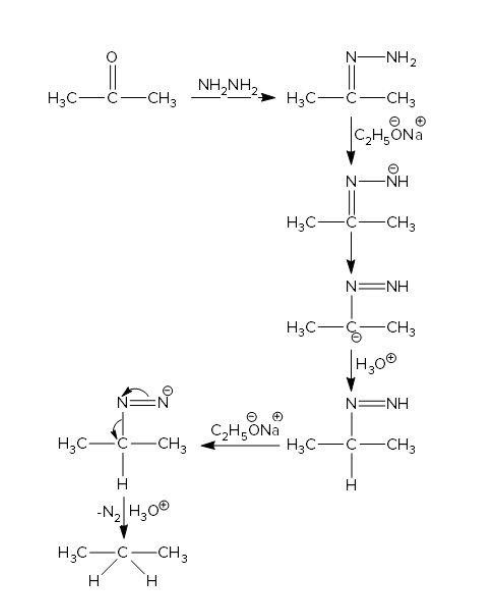
Let’s see the options,
The option (A) is Isopropyl alcohol which is not a carbonyl carbon and is not electrophilic in nature so this option is incorrect.
The option (B) is Butanaldehyde, it contains a carbonyl carbon but our product is propane so this option is also incorrect.
The option (C) is acetone which has a carbonyl carbon as well as it contains three carbon atom which can give propane as a product. Therefore, the correct option is (C).
The correct option is C.
Note:
The option containing alcohol could be simply ruled out since it is not electrophilic in nature.
