Question
Question: 
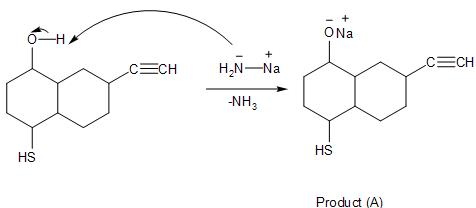
Product (A) is:
Solution
A base abstracts proton from the highly acidic substituent, due to the higher stability of the conjugate anion formed. Also, the strength of the base is accounted for by its conjugate acid strength.
Complete step by step answer:
In the given reaction, the presence of the base of sodium amide, it causes the deprotonation of the functional group with the highly acidic proton present on the ring. This is because the −NH2 anion is the conjugate base of the weak acid, NH3. Thus, making it a stronger base.
So, in the given compound, there is a hydroxyl group, the bisulphide group and the alkyne substituents attached to the ring. In order for the base to deprotonate the substituent, the most acidic proton will be favoured.
Then, in the hydroxyl group, with the oxygen being highly electronegative. It attracts the shared bond-pair electron, forming a negatively- charged anion formed which is highly stable. And making the hydrogen atom easily attracted to the base.
Whereas the bisulphide and the alkyne group being less acidic compared to the alcohol, as the sulphur and the carbon are less electronegative compared to oxygen. Thus, will not get deprotonated during the given reaction.
So, the reaction is as follows, with one mole of sodium amide. It only deprotonates the hydroxyl group, forming (A) as the final product.
Note: In the reaction, only one mole of the base is present. But if excess moles were present then the remaining two substituents would also have got deprotonated.
