Question
Question: 
What is true about that reaction?
(A) A is meso, 2,3-butandiol is formed by syn addition
(B) A is meso, 2,3-butandiol is formed by anti addition
(C) A is a racemic mixture of d and l, 2,3-butandiol is formed by anti addition
(D) A is a racemic mixture of d and l, 2,3-butandiol is formed by syn addition
Solution
Necessary requirement for a compound to be optically active is absence of all symmetry elements. Potassium permanganate always adds two -OH groups to alkene from the same side.
Complete answer:
Let’s find out the product first.
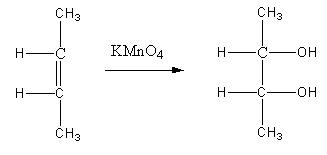
Here the product formed has a mirror plane that cuts the molecule into half.
So, any molecule that has this property is called a meso compound. Now even though the meso compound has a chiral carbon, the compound is not optically active. The reason is that elements of symmetry are present in the molecule. So, we can say that A is not a racemic mixture.
If the addition of two new groups in the alkene is from the same side, then the addition is called syn addition. We can see that in compound A -OH group is on the same side of the alkene. So it is a syn addition.
Hence the true answer is (A) A is meso, 2,3-butandiol is formed by syn addition.
Additional Information:
- Remember that being asymmetric is the primary requirement of the optically active compound. So, as in the case of meso compounds, even though the compound has chiral carbon, it is not optically active. This is because they have mirror planes present.
- If the addition of two new groups in the alkene is from the same side, then the addition is called syn addition.
- If the addition of two new groups in the alkene is from different sides, then the addition is called anti addition.
- Remember that Potassium Permanganate always does syn addition to alkenes and forms diols.
Note: Do not consider a meso compound optically active even though it contains a chiral carbon. It is possible that the compound contains chiral carbon but is not optically active. Remember that Potassium permanganate never does anti addition.
