Question
Question: 
In this reaction ‘X’ is
(1) HNO3
(2) O3
(3) O2
(4) KMnO4 (hot) solution
Solution
Looking towards the given reaction it seems that the redox reaction is happening here to give the product along with other mechanisms as well. The di-one formation from alkyne seems the reaction has an intermediate; a stable one.
Also, only one reagent can be present in the place of ‘X’ so, we would only have one correct answer.
Complete step by step answer:
Let us learn about ozonolysis as the formation of di-one from alkyne is possible when ozonolysis takes place.
Ozonolysis-
Ozonolysis is the organic chemical reaction where ozone is used to break the bonds of the unsaturated hydrocarbons. It is typically a redox reaction.
-With alkenes, ozonolysis gives alcohols, aldehydes, carboxylic acids or ketones.
-With alkynes, ozonolysis gives acid anhydrides or diketones (as we have in the given illustration).
Reaction-
Ozone is the most reactive allotrope of oxygen present in nature. The reaction of alkenes or alkynes with the ozone causes the oxidative breaking of unsaturated bonds.
The ozone breaks the pi-bond and sigma bond as well to form an ozonide. Then Zn dust is employed to break this oxygen linkage as Zn will form ZnO with oxygen.
Now, specifically let us see the ozonolysis of alkynes;
Ozonolysis is employed on alkynes to give acid anhydride or di-one as a final product. This mechanism can also be used to determine the position of the triple bond in an unknown alkyne.
Mechanism-
1. Ozonide formation- Alkyne reacts with ozone which causes the breakage of the alkyne.
2. Di-carbonyl compound formation- Zn along with H2O yields a di-carbonyl compound by removing the oxygen from the ozonide intermediate.
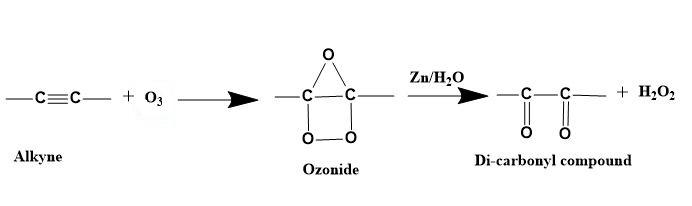
Illustration-
Here, 2-pentyne undergoes ozonolysis to give pentane-2, 3-dione.
Therefore, we can say that the ‘X’ in the given reaction is ozone i.e. O3.
So, the correct answer is “Option 2”.
Note: Do note that the ozonolysis mechanism varies with respect to the reactants i.e. alkene or alkyne. So, do not mix up the mechanisms.
