Question
Question: 
(A) 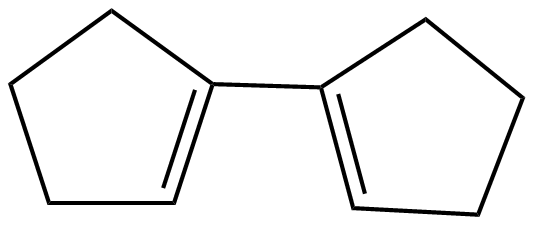
(B) 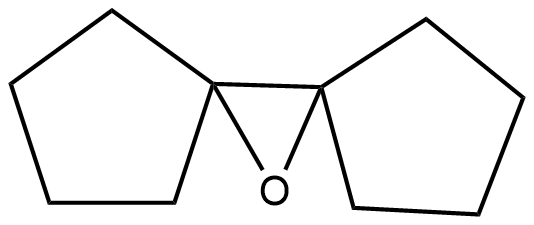
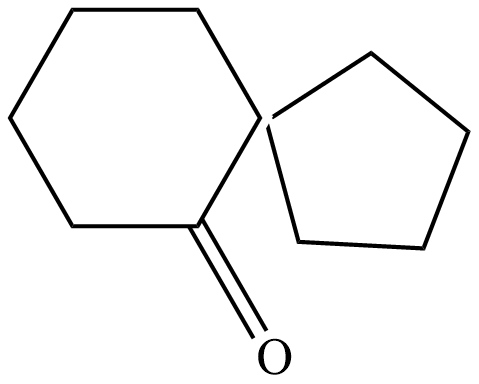
(C)
(D) 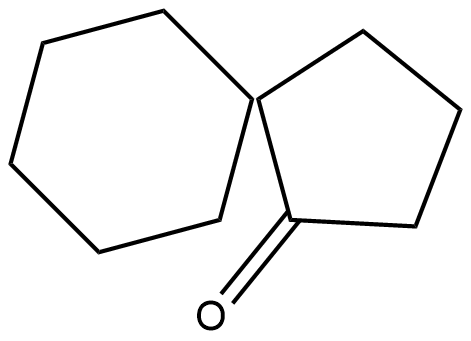
Solution
The given reaction is a dehydration of alcohol which is chemically named as Pinacol-pinacolone rearrangement reaction. When 1,2-diols are under heat and react with H+ and rearrange into a ketone group.
Complete step by step answer:
The IUPAC name of the given compound is 1,1'-Bi(cyclopentyl)-1,1'-diol.
The conversion of pinacols(1,2-glycols) to ketones or aldehydes by means of mineral acids or zinc chloride is known as pinacol-pinacolone rearrangement. In this reaction 1,1'-Bi(cyclopentyl)-1,1'-diol which is pinacol in this reaction and the product formed is pinacolone.
The given reaction is dehydration of 1,1'-Bi(cyclopentyl)-1,1'-diol under heat. The reaction mechanism is as follows.
Mechanism:
Step-1: protonation of 1,1'-Bi(cyclopentyl)-1,1'-diol.
In this step ,1'-Bi(cyclopentyl)-1,1'-diol convert into protonium ion by reacting with H+
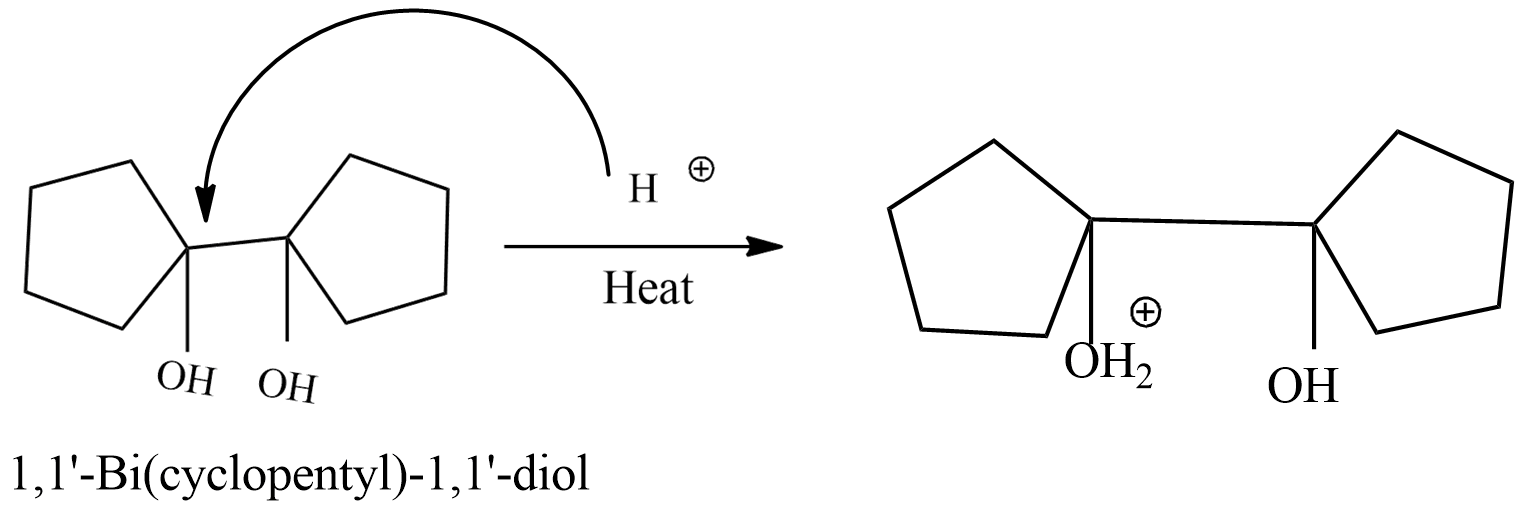
Step-2: formation of carbonium ion by loss of water.the oxonium which is formed in the first step will lose water molecules and form 1-hydroxy 1,1'-Bi(cyclopentyl) carbonium ion.
+ H2O
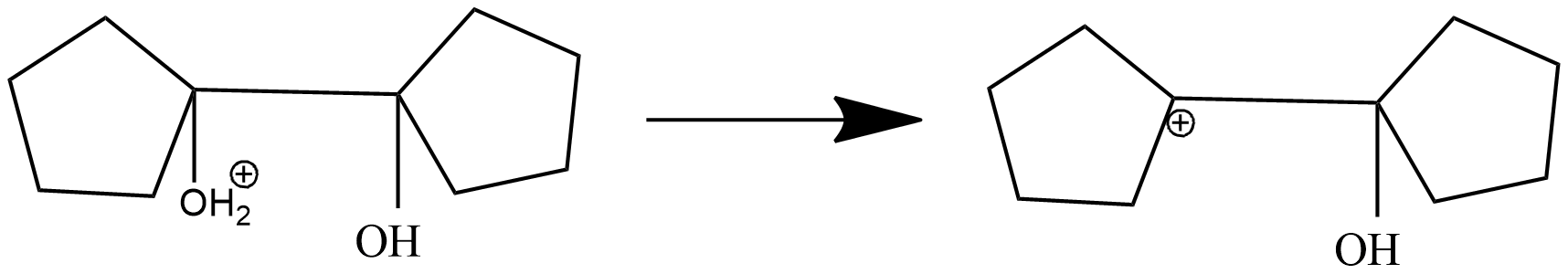
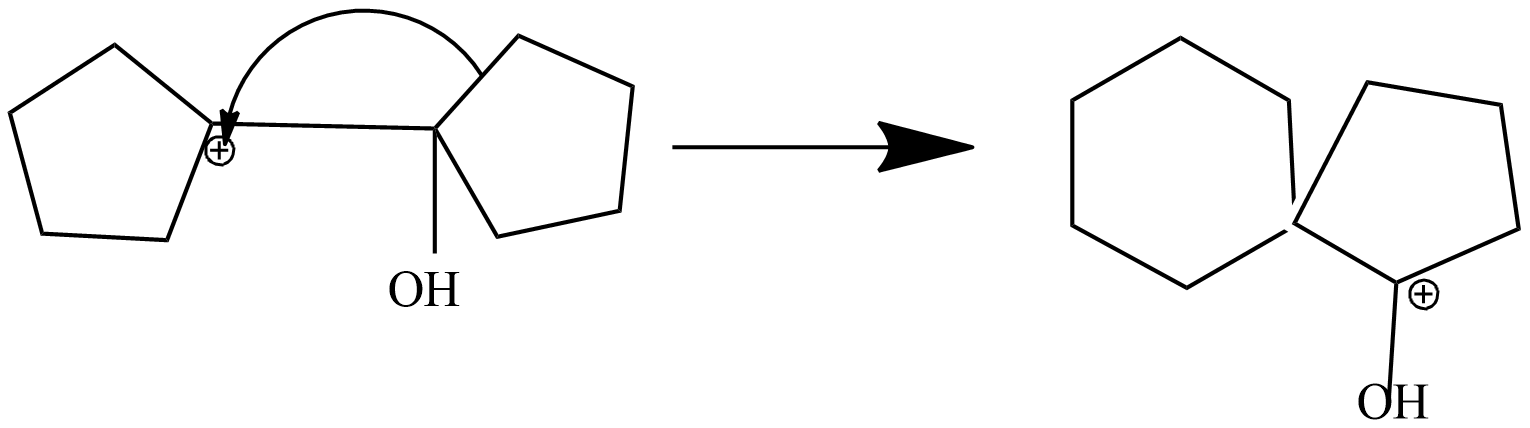
Step-3 : rearrangement of carbonium ion by 1,2-shift to give protonated ketone.
In this step, 1-hydroxy 1,1'-Bi(cyclopentyl) carbonium ion is rearranged into protonated ketone as shifts adjacent carbon to another carbon. Due to stable carbonium ion formation this rearrangement takes place. Subsequently, aryl group from the adjacent carbon migrates to the carbocation centre. The driving force for this arrangement step is believed to be the relative stability of the resulting oxonium ion.
Step-4: formation of ketone by loss of proton from protonated ketone
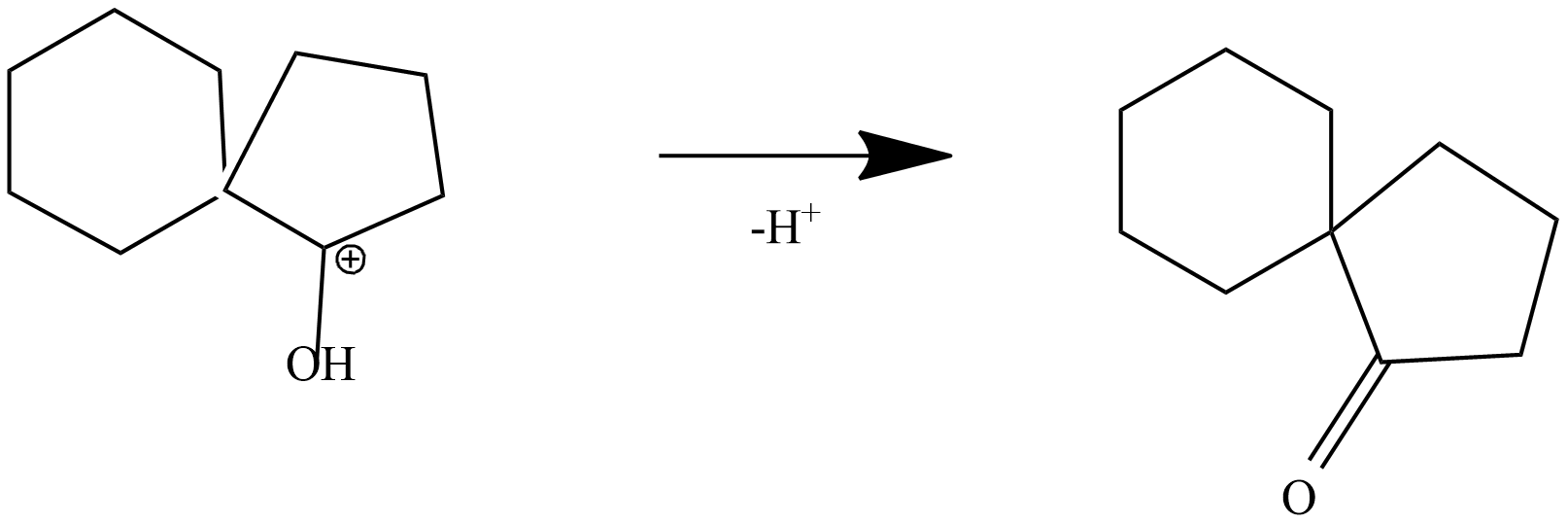
The product is a ketone product of 1,1'-Bi(cyclopentyl)-1,1'-diol.
Hence the correct answer is option D.
The final reaction is:
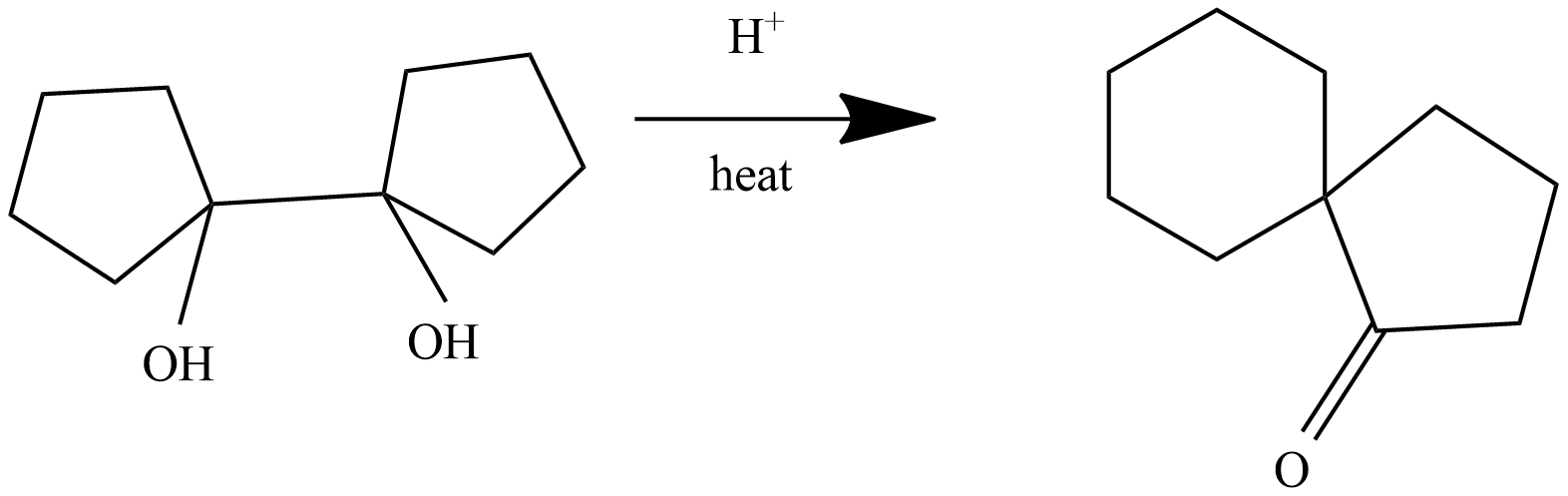
This reaction is anionic rearrangement, in which the migration group moves from a carbon atom to electron deficient carbon which has only six electrons in its valence shell.
In this reaction, the reactant 1,1'-Bi(cyclopentyl)-1,1'-diol is an example for 1,2-glycol and the migrating group which is an aryl group.
Note: The migration of alkyl groups in pinacol and pinacolone rearrangement like reaction occurs according to their usual migratory aptitude, i.e hydride > phenyl carbanion > tertiary carbanion > secondary carbanion>primary carbanion. Because every migrated group leaves by taking an electron pair with it.
