Question
Question: 
Indicate sigma and pi bonds in the above molecule.
Solution
Any single covalent bond which connects two atoms is called a sigma bond. A bond that involves overlapping of p-orbitals to form a multiple bond with adjacent carbon is called a pi bond.
Complete answer:
Let’s see the C-H bonds also in this molecule.
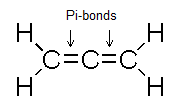
You can see two arrows pointing towards the carbon- carbon double bond. Both the arrows show a pi-bond.
So, we can say that there are two pi-bonds present in the molecule. Both are present in carbon-carbon double bonds as shown in the figure.
Now, sigma bonds are any single bonds between any two atoms. In double bonds also, one sigma bond is always present. Let’s see the sigma bonds in this molecule.
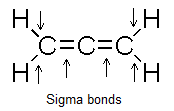
We can see that there are four C-H sigma bonds and two C-C sigma bonds present in the compound as shown in the figure. So, there are a total of six sigma bonds present in the compound.
Additional Information:
- Sigma bond formation occurs when two atoms share one electron each to form a two centre two electron covalent bond. It is denoted by σ-bond.
- Pi bond formation occurs when there is overlapping of orbitals between adjacent atoms and formation of multiple bonds. It is denoted by π-bond also.
Note: Do not forget to include C-H sigma bonds in the calculation of sigma bonds present in the compound as these bonds are often omitted while calculating the number of bonds. Remember that there is one sigma bond that is also present in a carbon-carbon double bond, do not forget to include it in the calculation also.
