Question
Chemistry Question on Bohr’s Model for Hydrogen Atom
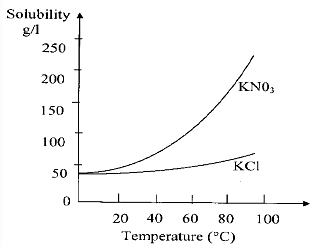 Given the solubility curves of KNO3 and KCl, which of the following statements is not true?
Given the solubility curves of KNO3 and KCl, which of the following statements is not true?
A
(A) At room temperature the solubility of KNO3 and KCl are not equal.
B
(B) The solubilities of both KNO3 and KCl increase with temperature.
C
(C) The solubility of KCl decreases with temperature.
D
(D) The solubility of KNO3 increases much more compared to that of KCl with increase in temperature.
Answer
(C) The solubility of KCl decreases with temperature.
Explanation
Solution
Explanation:
We can see from the curve of both the compounds:Solubility increases as temperature increase with different rate. Therefore, statement in option (C) is incorrect as it says solubility decreases with increase in temperature.Hence, the correct option is (C).
