Question
Question: How would you show the bonding of Hydrogen and Oxygen using Lewis Dot diagrams?...
How would you show the bonding of Hydrogen and Oxygen using Lewis Dot diagrams?
Solution
The hydrogen and oxygen combine to form a molecule of water. The electrons are arranged in between the two hydrogens and one oxygen atom.
Complete step by step answer:
Hydrogen is an element in the periodic table with atomic number 1 and electronic configuration 1s1. It has only one electron available for combination with other elements. The valence shell of a hydrogen atom is 1.
Oxygen is an element in the periodic table with atomic number 8 and electronic configuration[He]2s22p4. The valence shell of an oxygen atom is 2 having six electrons in the outermost shell with two in 2s and four in 2p orbitals.
The valency of hydrogen atom is one and that of oxygen atom is two. It is because hydrogen needs one electron to reach the nearest noble gas configuration and the oxygen atom needs two electrons to complete the octet.
Thus for completing the octet of hydrogen two electrons are shared between a hydrogen atom and an oxygen atom. But the total number of electrons in that case is7. So the oxygen atom bonds with another hydrogen atom by sharing an electron pair. Thus there are 2 valence electrons on each of the hydrogen atoms and 8 valence electrons on the oxygen atom.
Hence the Lewis Dot diagram showing the bonding of Hydrogen and Oxygen is as follows:
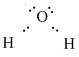
Note:
Every element in the Lewis dot structure must have a complete octet of electrons. The central atom is sp3 hybridized with two lone pairs and two bond pairs. The molecular geometry of the molecule is bent and the electronic geometry is tetrahedral. The ∠H−O−H is 104.5∘ .
