Question
Question: How would you predict the ideal bond angles(s) around each central atom in this molecule?  around each central atom in this molecule?
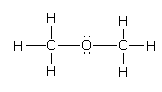
Solution
Then by using the VSEPR theory determine the electron geometry and molecular geometry of the compound.The full form of the VSEPR theory is the Valence Shell Electron Pair Repulsion Theory in which the electron and the molecular geometry of the molecule are different because of the presence of the lone pairs of electrons around the central atom.
Complete step-by-step answer: Here, the compound given is dimethoxy ether whose structure is as follows:Both methyl groups are in the same environment, the carbon of the methyl group is surrounded by three hydrogen atoms and one oxygen atom.There are four bond pairs of an electron and zero lone pairs of electrons on the carbon atom. It indicates carbon is sp3 hybridized and hence, all H−C−H and H−C−O bond angles are 109.5o.
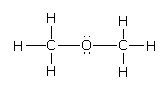
Now, if oxygen is the central atom then there are two bond pairs of an electron and two lone pairs of electrons.As per the VSEPR, the electron geometry is tetrahedral. But because of the presence of the two lone pairs, there is repulsion between the lone pairs and the bond pairs.This repulsion leads to a decrease in the tetrahedral bond angle to 104o to 106o like in the case of the water.
Note: The electron geometry considers the all electrons pair that is bonding as well as lone pairs of the electrons.While determining the molecular geometry that is the shape of the molecule only bonding electron pairs are considered.The repulsion between lone pairs and the bond pairs affect the bond angle between the molecules.The order of the repulsion is as follows:
lonepair - lonepair > lonepair - bondpair > bondpair - bondpair
